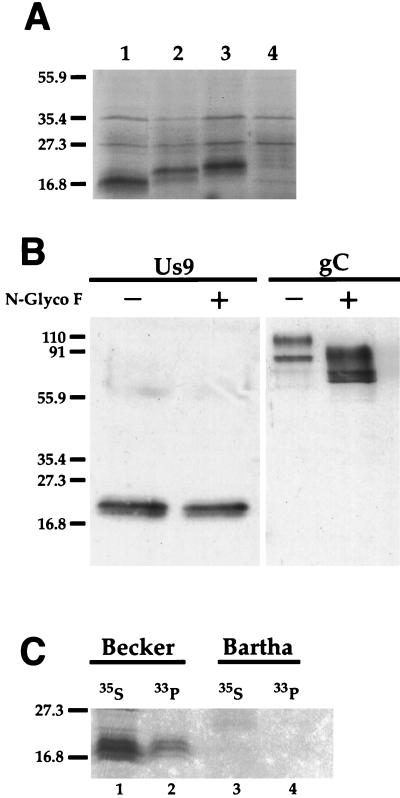
FIG. 4.
Us9 posttranslational modifications. (A) In vitro translation of Us9, Us9 M9A, and Us9-HA-tagged constructs. Plasmids pAB7 (lane 1), pAB19 (lane 2), and pAB11 (lane 3) were transcribed and translated in vitro in the presence of [35S]methionine by a rabbit reticulocyte lysate procedure. The bands migrating at approximately 27 and 35 kDa represent nonspecific translation products, as they are also detected during in vitro translation of the parental vector alone (pcDNA1/Amp) (lane 4). (B) N-glycosidase F treatment of Us9. PRV Be-infected cell lysates were incubated in either the presence (+) or absence (−) of N-glycosidase F (N-Glyco F). The treated lysates were separated on an SDS–12.5% polyacrylamide gel, electroblotted onto nitrocellulose, and analyzed by Western blotting with Us9 or gC antiserum. (C) Analysis of Us9 phosphoforms. PRV Be (lanes 1 and 2)- or Ba (lanes 3 and 4)-infected PK15 cells were radiolabeled overnight in the presence of either [35S]methionine-cysteine (lanes 1 and 3) or [33P]orthophosphate (lanes 2 and 4). Cellular extracts were prepared at 16 h postinfection and subjected to immunoprecipitation with Us9 antiserum. All of the immunoprecipitated products were analyzed by electrophoresis on an SDS–12.5% polyacrylamide gel followed by autoradiography. Positions of molecular mass markers (kilodaltons) are indicated on the left.