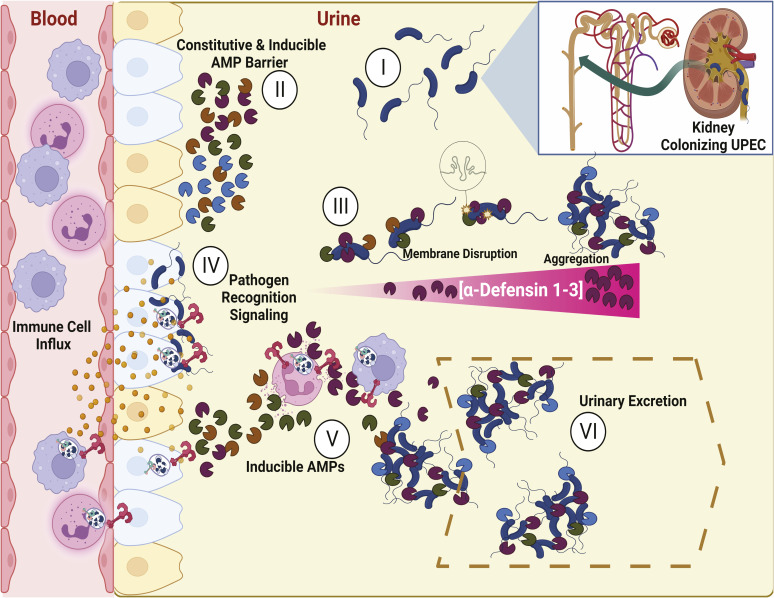
Figure S6. Proposed mechanism of kidney α-Defensin 1-3 gene dose–dependent protection against uropathogenic E. coli (UPEC) infection and inflammation.
(I) Colonizing UPEC ascends ureters and infects the distal kidney collecting ducts. (II) Collecting duct epithelial cells produce a constitutive and inducible repertoire of antimicrobial peptides (AMPs) that form a barrier against invasive uropathogens. Variable levels of α-Defensin 1-3 during infections are expressed proportionally to the harbored host DEFA1A3 gene copy number. (III) Alone and/or in concert with expressed AMPs, α-Defensin 1-3 induce membrane disruption and aggregation in a dose-dependent manner. (IV) Invasion of UPEC into the kidney leads to pro-inflammatory signaling and cellular influx via recognition of bacterial ligands through Toll-like receptors. (V) Induction of renal and extrarenal α-Defensin 1-3 from intercalated cells and neutrophils leads to agglutination of UPEC and associated components driving attenuated Toll-like receptor activities. (VI) Resulting interactions from the induction of α-Defensin 1-3 in concert with collecting duct–derived AMPs drive fine-tuning of pro-inflammatory cell infiltration, orchestrated bacterial damage, and subsequently excretion of agglutinated UPEC via urinary flow.