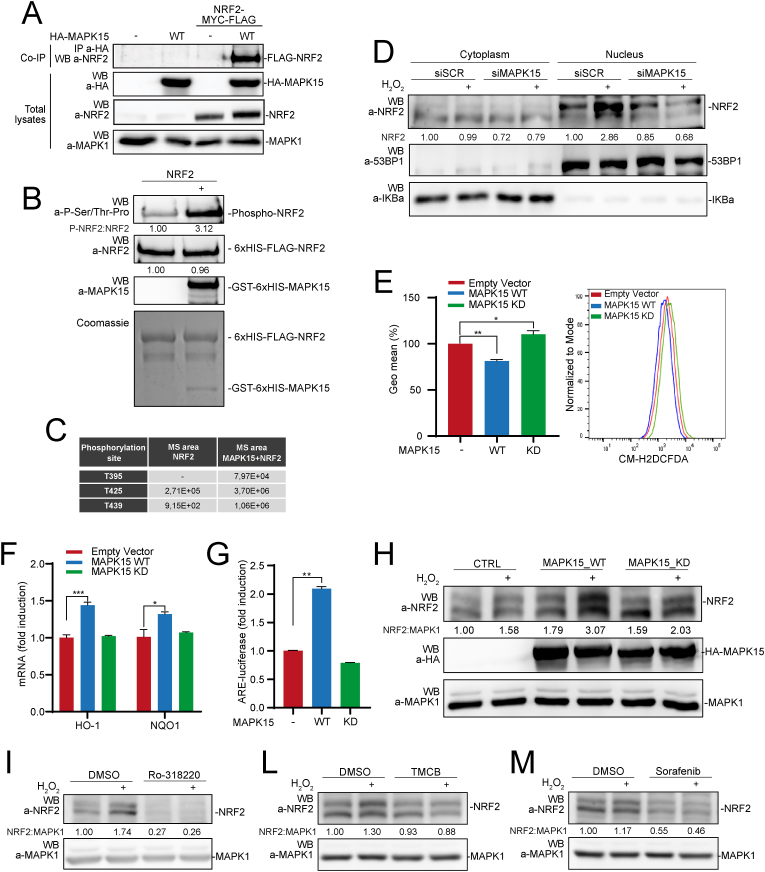
Fig. 3.
MAPK15 directly phosphorylates NRF2 and regulates its nuclear translocation. (A) 293T cells were transiently transfected with NRF2-MYC-FLAG plus empty vector or HA-MAPK15_WT. After 24 h, lysates were immunoprecipitated with anti-HA antibodies and subjected to SDS-PAGE followed by WB, to detect coimmunoprecipitated NRF2 protein. Total lysates were also analyzed for expression of indicated proteins. One experiment, representative of 3 independent experiments, is shown. (B) NRF2 protein purified from bacteria was subjected to kinase assay in presence or absence of recombinant, bacterially purified MAPK15. Samples from kinase reactions were resolved by SDS-PAGE, and NRF2 phosphorylation was demonstrated by WB using specific anti-phospho-Ser/Thr-Pro antibodies. Coomassie staining and WB analysis (with indicated antibodies) of kinase reactions were used as loading controls. (C) Kinase reactions prepared as in (B) were resolved by Mass Spectrometry analysis, to determine the identity and amounts of phosphorylated residues. (D) 293T cells were transfected with siSCR or siMAPK15. After 72 h, they were treated with 300 μM H2O2 for 1 h and then subjected to nucleo-cytoplasmic fractionation. Amounts of NRF2 in the different fractions were analyzed by WB. Normalization of cytosolic and nuclear lysates was performed by WB, using anti-IKBα and anti-53BP1 antibodies, respectively. One experiment, representative of 3 independent experiments, is shown. Densitometric analysis of bands is shown. (E) Representative Fluorescence-Activated Cell Sorting (FACS) histograms or corresponding Geometric Mean Fluorescent Intensity (GeoMFI) bars of CM-H2DCFDA (1 μM) fluorescence 24 h after 293T cells transfection using empty vector, MAPK15_WT or MAPK15_KD plasmids. Bars represent the average ± S.D. of 3 independent experiments (n = 3). (F) RT-qPCR was used to monitor mRNA expression of NQO1 and HO-1 in 293T cells transiently overexpressing empty vector, MAPK15_WT or MAPK15_KD. (G) 293T cells were co-transfected with ARE luciferase reporter vector plus empty vector or MAPK15_WT or MAPK15_KD. Twenty-four hours after transfections, samples were lysed and the luciferase activity was measured in cell extracts. Data are represented as fold induction of the normalized luciferase activity with respect to control cells transfected with GFP. All luciferase results represent the average ± S.D. of three independent experiments. All samples were read in triplicate. (H) 293T cells were transiently co-transfected with NRF2-MYC-FLAG plus empty vector or MAPK15_WT or MAPK15_KD. After 24 h, samples were treated with 300 μM H2O2, for 1 h. Lysates were analyzed by WB with indicated antibodies. One experiment, representative of 3 independent experiments, is shown. Densitometric analysis of bands is indicated. (I) 293T cells were treated with vehicle or 2 μM Ro-318220, for 6 h. During the last hour of Ro-318220 treatment, samples were treated with 300 μM H2O2, for 1 h. Then samples were collected and subjected to SDS-PAGE followed by WB. One experiment, representative of 3 independent experiments, is shown. Densitometric analysis of bands is shown. (L) Same as in (I), but cells were treated with vehicle or TMCB 10 μM, for 24 h. (M) Same as in (I), but cells were treated with vehicle or Sorafenib 10 μM, for 6 h.