Abstract
Background and Aims
Available data on continuous rhythm monitoring by implantable loop recorders (ILRs) in patients with Brugada syndrome (BrS) are scarce. The aim of this multi-centre study was to evaluate the diagnostic yield and clinical implication of a continuous rhythm monitoring strategy by ILRs in a large cohort of BrS patients and to assess the precise arrhythmic cause of syncopal episodes.
Methods
A total of 370 patients with BrS and ILRs (mean age 43.5 ± 15.9, 33.8% female, 74.1% symptomatic) from 18 international centers were included. Patients were followed with continuous rhythm monitoring for a median follow-up of 3 years.
Results
During follow-up, an arrhythmic event was recorded in 30.7% of symptomatic patients [18.6% atrial arrhythmias (AAs), 10.2% bradyarrhythmias (BAs), and 7.3% ventricular arrhythmias (VAs)]. In patients with recurrent syncope, the aetiology was arrhythmic in 22.4% (59.3% BAs, 25.0% VAs, and 15.6% AAs). The ILR led to drug therapy initiation in 11.4%, ablation procedure in 10.9%, implantation of a pacemaker in 2.5%, and a cardioverter-defibrillator in 8%. At multivariate analysis, the presence of symptoms [hazard ratio (HR) 2.5, P = .001] and age >50 years (HR 1.7, P = .016) were independent predictors of arrhythmic events, while inducibility of ventricular fibrillation at the electrophysiological study (HR 9.0, P < .001) was a predictor of VAs.
Conclusions
ILR detects arrhythmic events in nearly 30% of symptomatic BrS patients, leading to appropriate therapy in 70% of them. The most commonly detected arrhythmias are AAs and BAs, while VAs are detected only in 7% of cases. Symptom status can be used to guide ILR implantation.
Keywords: Brugada syndrome, Loop recorder, Rhythm monitoring, Syncope, Ventricular arrhythmias, Brady-arrhythmias, Atrial arrhythmias, Sudden cardiac death
Structured Graphical Abstract
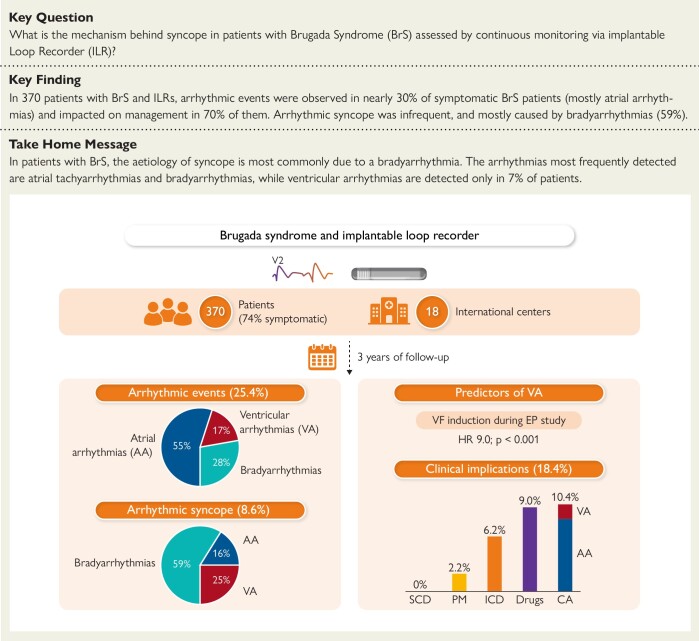
Structured Graphical Abstract.
In our large cohort of 370 Brugada syndrome (BrS) patients from 18 international centers monitored continuously with an implantable loop recorder (ILR) for 3 years, 25.4% of the patients experienced an arrhythmic event (per-patient analysis). Specifically, 16% experienced an atrial arrhythmia, 5% a ventricular arrhythmia (VA), and 8% a bradyarrhythmia (per-event analysis). The occurrence of arrhythmic syncope in the whole cohort was 8.6% (32/370). The induction of ventricular fibrillation (VF) during an electrophysiological (EP) study emerged as the only predictor of VAs in this cohort. The clinical implications are detailed in the lower right part of the figure (SCD, sudden cardiac death; PM, pacemaker; ICD, implantable cardioverter-defibrillator; Drugs include anti-arrhythmic drugs and anticoagulants; CA, catheter ablation for atrial arrhythmias or VAs).
See the editorial comment for this article ‘Can an implantable loop recorder improve risk stratification and appropriate management in Brugada syndrome?’, by F. Gaita et al., https://doi.org/10.1093/eurheartj/ehae136.
Introduction
Brugada syndrome (BrS) is an inherited arrhythmia syndrome, characterized by an increased risk of ventricular arrhythmias (VAs) and sudden cardiac death (SCD).1 Syncope and palpitations are common symptoms among patients with BrS, occurring in ∼30% of cases.2–4 The cause of symptoms in BrS is highly variable, ranging from neuro-mediated reflex to a broad range of arrhythmias.
According to the last European Society of Cardiology (ESC) guidelines, an implantable cardioverter-defibrillator (ICD) implantation for primary prevention should be considered in patients with BrS and history of arrhythmic syncope (class IIa, level of evidence C).5–7 However, defining the arrhythmic origin of syncope is challenging and relies on the patient’s description of the event.8 Furthermore, both brady- and tachyarrhythmias of non-ventricular origin can cause a syncopal episode.8
To effectively manage patients with BrS, it is essential to mitigate the risk of SCD, avoiding at the same time over-treatment and complications from ICDs.9 The use of implantable loop recorders (ILRs) has emerged as a valuable tool in this context and carries a class IIa recommendation in patients with syncope of undetermined origin.5–7 However, the evidence supporting this recommendation is modest, derived from few small (<50 patients) single-centre studies and supported by expert opinion (level of evidence C).8,10,11 Further insight is therefore needed to enhance our understanding of ILR utilization, to refine the diagnostic assessment of symptoms and syncope, and ultimately to optimize ICD indications in BrS patients.
The primary objective of this study was to evaluate, in a large multicenter cohort of symptomatic and asymptomatic BrS patients with ILRs, the indications, findings, and clinical implications of ILR implantation. Second, the study aimed to accurately identify the precise aetiology of syncopal episodes. Finally, we aimed to assess predictors of arrhythmias in BrS patients with ILRs.
Methods
Study design
This study is a retrospective multi-centre study investigating the use of ILRs in patients with BrS. The study was approved by the local ethic committee (Swiss Ethics, approval number: BASEC 2019-00754/CE 3476) and conforms to the Declaration of Helsinki. All patients provided written informed consent. The datasets generated and/or analysed during the current study are not publicly available to maintain patient confidentiality but are available from the corresponding author on reasonable request and after the agreement of all the co-authors.
Patient population—inclusion and exclusion criteria
The study included patients who met all of the following inclusion criteria:
Diagnosis of BrS, based on the last 2022 ESC guidelines, with a Shanghai score ≥ 3.5.5,7,12 Specifically, patients were included in the presence of a spontaneous type 1 electrocardiogram (ECG) [coved-type ST-segment elevation ≥2 mm in ≥1 right precordial leads (V1–V3) positioned in the 4th, 3rd, or 2nd intercostal space] or drug/fever-induced Brugada type 1 ECG plus one of the following: (i) documented ventricular fibrillation (VF) or polymorphic ventricular tachycardia (VT), (ii) arrhythmic syncope or nocturnal agonal respiration, (iii) family history of SCD at 45 years old with negative autopsy, and (iv) family history of BrS.
Implantation of an ILR after the diagnosis of BrS, with a follow-up of at least 12 months. Patients with <50% of required baseline data were excluded from the study, as well as patients with previously diagnosed arrhythmias or under anti-arrhythmic medications.
Data collection and definitions
Patient data, including the indication for ILR implantation and demographic and clinical information, [e.g. Shanghai score, family history, ECG parameters, genetic test, and electrophysiological study (EPS)] were collected and analysed.
Patients were defined as symptomatic if the reason for ILR implantation was one of the following: (i) syncope or (ii) palpitations. The suspected syncopal aetiology was defined as follows: (i) arrhythmic syncope: abrupt onset without prodromal symptoms and triggers, short duration, and prompt recovery; (ii) vasovagal: syncope associated with typical triggers (e.g. pain, fear, long standing), and typical progressive prodromes (pallor, sweating, nausea); (iii) unexplained/unknown origin: when the clinical features did not allow to classify into one of the above forms.6,8 Tilt table test, Schellong test, and other investigations to achieve a comprehensive characterization of the syncopal episodes before ILR implant was left at the treating physician’s discretion, according to guidelines.6,8
During follow-up, arrhythmic events were collected and divided into three categories, based on current ESC guidelines:13–15
Bradyarrhythmias (BAs) of non-vaso-vagal origin, including (i) sinus arrest of 3 to 6 s if symptomatic, or longer than 6 s if asymptomatic, (ii) advanced atrio-ventricular (AV) block (second-degree type II AV block or third-degree AV block). The vasovagal origin of each episode was assessed and ruled out based on typical triggers and prodromes; and sinus node slowing preceding or occurring concurrently with AV block.
atrial arrhythmias (AAs), including (i) atrial fibrillation (AF)/flutter (AFL) longer than 6 min, (ii) atrial tachycardia or paroxysmal supraventricular tachycardia (PSVT) longer than 30 s;
VAs, including (i) symptomatic non-sustained VT (NSVT, 3 or more consecutive ventricular heartbeats at a rate >100 bpm and lasting <30 s in duration), (ii) monomorphic sustained VT, (iii) polymorphic VT or VF.13–15 All participating centers routinely performed remote monitoring of the device, and patients were systematically contacted within 24–48 h after the arrhythmic event to facilitate the correlation with symptoms. All ILR events were reviewed and adjudicated by expert electrophysiologists. Arrhythmic events were categorized based on: date of the first event, frequency of the event, and correlation with the initial symptoms. Data on mortality and cause of death were also recorded.
Endpoints
The primary endpoint of the study was the incidence of arrhythmic events in BrS patients with ILRs according to the symptom status, and the correlation with the initially reported symptoms. The secondary endpoint was the clinical impact of the ILR via a composite endpoint, including at least one of the following: (i) initiation of drug therapy (anti-arrhythmic drugs and/or anticoagulants), (ii) atrial or ventricular ablation procedure, (iii) pacemaker (PM) or ICD implantation. Another secondary endpoint was the identification of independent predictors of VAs.
Statistical analysis
Continuous variables are presented as mean ± standard deviation when normally distributed or otherwise median and interquartile range. Comparisons between groups were undertaken with parametric (Student’s t-test) or non-parametric (Mann–Whitney U test) test, respectively. The comparison between categorical variables was performed with the χ2 test and the Fisher’s exact test, as indicated. Event-free survival probability was estimated using the Kaplan–Meier method. Cox regression analysis [hazard ratios (HR) and 95% confidence interval (CI)] and logistic regression analysis were used to estimate the association between baseline characteristics and arrhythmic events. A two-sided P-value <.05 was considered statistically significant. Statistical analysis was performed using SPSS 23.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
The study population included 370 patients with BrS (mean age 43.5 ± 15.9 years, 33.8% female), who underwent ILR implantation between 2013 and 2022 in 18 international centers. All participating centers were University Hospitals and 66% of them had a dedicated Unit on inherited arrhythmia syndrome. Baseline characteristics are reported in Table 1.
Table 1.
Baseline characteristics of the participants (n = 370)
| Characteristics | |
|---|---|
| Age, years, mean (SD) | 43.5 ± 15.9 |
| Female sex, n (%) | 125 (33.8) |
| Proband status, n (%) | 229 (61.9) |
| Family history of BrS, n (%) | 111 (30.1) |
| Family history of SCD, n (%) | 101 (27.3) |
| Spontaneous Brugada type 1 ECG, n (%) | 150 (40.5) |
| Shanghai score, median (IQR) | 4 (3.5–5.5) |
| Genetic test, n (%) | 201 (54.3) |
| SCN5A P/LP variant, n (%) | 58/201 (29.4) |
| VF induction during EP study, n (%) | 18/224 (8.0) |
| HV during EP study, ms mean (SD) | 42.1 ± 6.2 |
| cSNRT during EP study, ms, mean (SD) | 421.3 ± 49.1 |
| Symptoms before ILR, n (%) | |
| Syncope | 190 (51.4) |
| Palpitations | 84 (22.7) |
| Any symptoms (all of the above) | 274 (74.1) |
| No symptoms | 96 (25.9) |
| Syncope characterization before ILR | |
| Suspected arrhythmic, n (%) | 9 (4.7) |
| Unexplained, n (%) | 181 (95.3) |
| Time from syncope to ILR, months, median (IQR) | 1.9 (0.3–18.2) |
Continuous variables are shown as mean ± SD or median (IQR). Discrete variables are presented as n (%).
Abbreviations: AF, atrial fibrillation; EP, electrophysiological; ILR, implantable loop recorder; IQR, interquartile range; SCD, sudden cardiac death; SD, standard deviation; VF, ventricular fibrillation; P, pathogenic, LP, likely pathogenic; cSNRT, corrected sinus node recovery time.
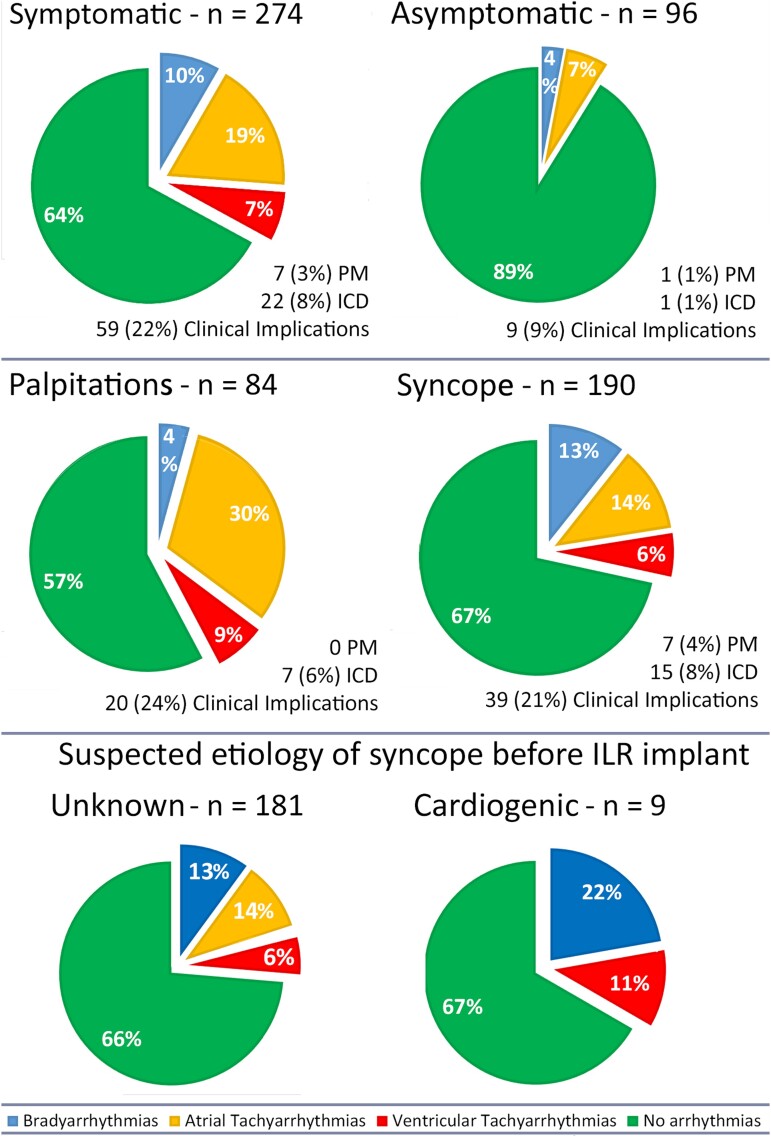
Overall, 274 patients (74.1%) were implanted due to symptoms (69.3% syncope, 30.7% palpitations), while the remaining 96 (25.9%) were asymptomatic. In patients with syncope, the suspected aetiology was undetermined in 181 (95.3%) and arrhythmic in 9 (4.7%—in these patients, an ICD was considered but refused by the patient; therefore, an ILR was implanted). The reason for ILR implant in asymptomatic patients was: cryptogenic stroke (4%), polymorphic NSVT induced at EPS (11%), NSVT or subclinical bradyarrhytmias registered at Holter monitor (23%), patient’s request of continuous rhythm monitoring (30%), close follow-up in patients refusing EPS (32%).
More than one-third of the patients (40.5%) had a spontaneous Brugada type 1 ECG and 27.3% had a family history of SCD. A genetic test was performed in 201 (54.3%) patients, and a SCN5A pathogenic/likely pathogenic (LP) variant was found in 58 (29.4%). An EPS with programmed ventricular stimulation (PVS) up to 2 extra-stimuli was performed in 224 patients (60.5%) with VF (n = 14) or sustained polymorphic ventricular tachycardia (n = 4) being induced in 18 (5.6%). In all of these patients, an ICD was considered but refused by the patient; therefore, an ILR was implanted.
Arrhythmic events at follow-up
Over a median follow-up of 33.7 months (14.9–52.6), 114 arrhythmic events were recorded in 94 patients (25.4%), with a yearly arrhythmia incidence rate of 8.6% (95% CI 3.6%–35.3%) (Structured Graphical Abstract).
Specifically, 32 patients (8.6%) experienced a significant bradyarrhythmia [6 advanced AV block and 29 sinus arrest (mean duration 11.3 ± 4.1 s)], 58 patients (15.7%) atrial tachyarrhythmias (40 AF and 19 PSVT). Twenty patients (5.4%) experienced symptomatic VAs (14 NSVT, 4 sustained monomorphic VT, 1 polymorphic VT, and 1 VF) with a yearly incidence rate of 1.1% (0.9%–2.2%). The mean duration of NSVT was 17.4 ± 4.7 s with a mean number of ventricular complexes of 52.2 ± 14.1. No patient died during follow-up, but one patient was successfully resuscitated from an episode of VF.
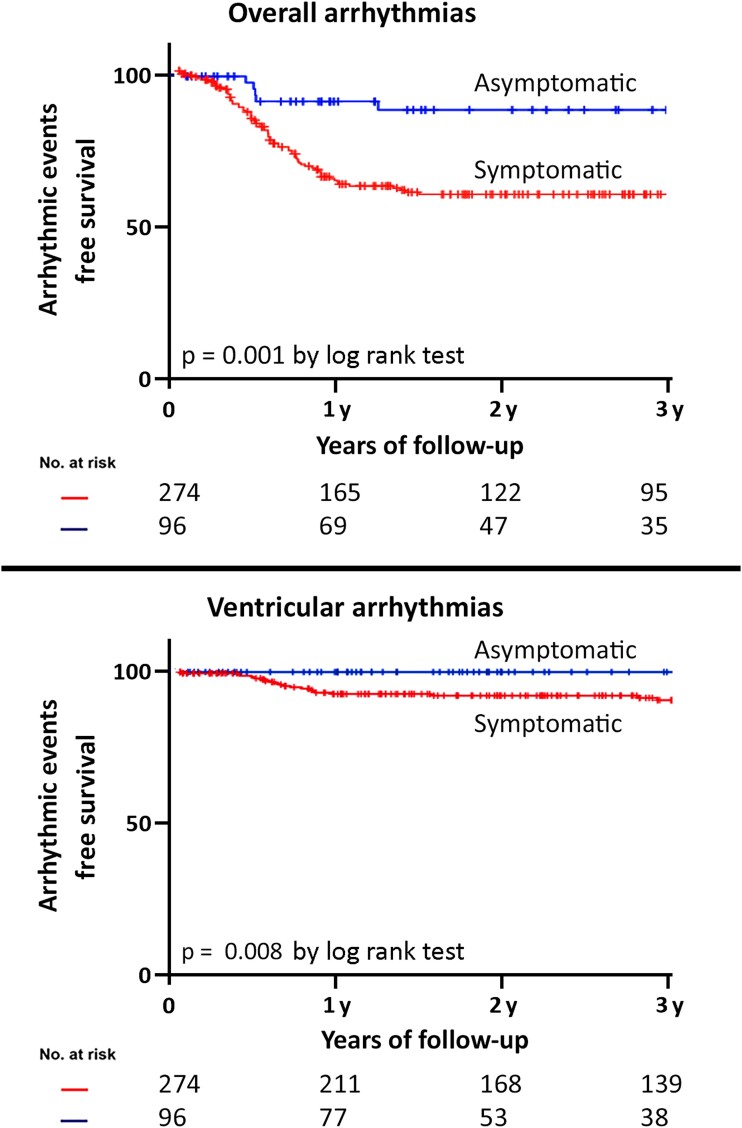
Among symptomatic patients, the arrhythmic event rate was 30.7% (Figures 1 and 2), significantly higher if compared with asymptomatic patients (10.0%, P = .001, Figure 1). A correlation between symptoms before ILR implantation and arrhythmia detection was found in more than half of patients (58.6%). The type of arrhythmia, stratified based on the ILR indication, is shown in Figure 2, with 6% of the ventricular events being recorded among patients implanted for syncope and 9% among patients implanted for palpitations.
Figure 1.
Arrhythmic event free-survival stratified according to symptom status before implantable loop recorder implant. Top panel: overall arrhythmias (atrial tachyarrhythmias, ventricular arrhythmias, and bradyarrhythmias); bottom panel: ventricular arrhythmias
Figure 2.
Type of arrhythmia detected at implantable loop recorder monitoring, stratified according to symptom status at baseline
Characterization of syncope
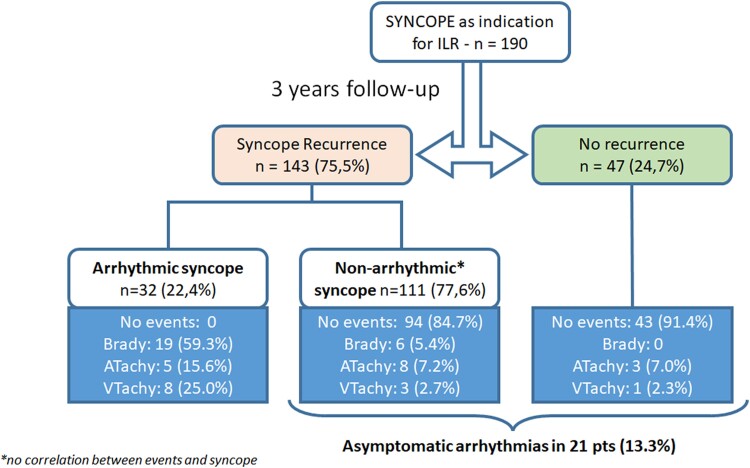
At 3-year follow-up, syncope recurrence was experienced by 143 patients (75.3%) (Figure 3). Of them, a symptom-correlated arrhythmia was found in 22.4% (arrhythmic syncope), while no arrhythmia was documented in the remaining 77.6%. A bradyarrhythmia was responsible for the syncopal recurrence in the majority (59.3%) of patients, while a VA was documented in 25% of patients. In the remaining 15.7% of patients, the cause of syncope was an atrial arrhythmia with fast ventricular response (Structured Graphical Abstract, Figure 3). Among patients with suspected arrhythmic syncope at baseline (nine patients), the arrhythmic event rate was 33%, with 22% experiencing advanced AV block and 11% experiencing NSVT (Figure 1). Notably, an asymptomatic arrhythmia was recorded in 13.3% of patients with previous syncope (Figure 3).
Figure 3.
Characterization of syncope by implantable loop recorder. Details on the arrhythmic event are as follows: Bradyarrhythmias (n = 19, 59.3%): atrio-ventricular block (n = 4), Sinus arrest (n = 15). Atrial arrhythmias (n = 5, 15.7%): Atrial fibrillation (n = 5). Ventricular arrhythmias (n = 8, 25.0%): sustained ventricular tachycardia (n = 3), ventricular fibrillation or polymorphic ventricular tachyarrhythmias (n = 2), non-sustained ventricular tachyarrhythmias (n = 3)
Clinical implications
The composite secondary endpoint was reached in 68 patients (18.4%). An ICD or PM was implanted in 23 (6.2%) and 8 (2.2%) patients, respectively. Among patients who underwent ICD implantation, six had a positive EPS (26%) before ILR implantation.
Drug therapy was initiated for 34 patients (9.0%), including quinidine for 9 patients, class III anti-arrhythmic drugs for 15 patients, and oral anticoagulants for 23 patients. Additionally, 37 patients (10.4%) were referred for catheter ablation (CA): 31 patients underwent AAs ablation (6 atrioventricular nodal reentrant tachycardia/atrioventricular reentrant tachycardia ablation, 25 AF ablation) and 6 patients underwent VA ablation (3 monomorphic VT, 1 polymorphic VT, and 1 VF) (Structured Graphical Abstract). In symptomatic patients, ILR led more frequently to significant clinical implications as compared to asymptomatic patients (Table 2).
Table 2.
Follow-up outcomes comparison between symptomatic and asymptomatic patients
| All pts. (n = 370) | Pts. w symptoms (n = 274) | Pts. w/o symptoms (n = 96) | HR | Lower 95%CI | Upper 95% CI | P-value | |
|---|---|---|---|---|---|---|---|
| Arrhythmic events | |||||||
| Overall arrhythmias | 94 (25.4%) | 84 (30.7%) | 10 (10.0%) | 3.1 | 1.6 | 5.9 | .001 |
| Brady-arrhythmic events | 32 (8.6%) | 28 (10.2%) | 4 (4.2%) | 2.2 | 0.7 | 6.4 | .14 |
| Atrial tachyarrhythmias | 58 (15.7%) | 51 (18.6%) | 7 (7.3%) | 2.5 | 1.1 | 5.5 | .022 |
| Ventricular tachyarrhythmias | 20 (5.4%) | 20 (7.3%) | 0 | – | – | – | – |
| Arrhythmic death | 0 | 0 | 0 | – | – | – | – |
| All patients (n = 370) | Pts. w symptoms (n = 274) | Pts. w/o symptoms (n = 96) | OR | Lower 95%CI | Upper 95% CI | P-value | |
|---|---|---|---|---|---|---|---|
| Clinical implications | |||||||
| PM implantation | 8 (2.2%) | 7 (2.5%) | 1 (1.0%) | 2.5 | 0.3 | 20.6 | .39 |
| ICD implantation | 23 (6.2%) | 22 (8.0%) | 1 (1.0%) | 8.3 | 1.1 | 62.4 | .040 |
| Drug therapy initiation | 34 (9.0%) | 31 (11.4%) | 3 (3.0%) | 1.0 | 0.9 | 1.1 | .86 |
| Atrial arrhythmias ablation | 31 (8.4%) | 24 (8.8%) | 7 (7.3%) | 2.5 | 0.8 | 7.8 | .12 |
| Ventricular arrhythmias ablation | 6 (2.0%) | 6 (2.2%) | 0 | – | – | – | – |
| Overall clinical impact | 68 (18.4%) | 59 (21.5%) | 9 (9.4%) | 2.7 | 1.3 | 5.6 | .010 |
Univariate COX regression (upper part) and logistic regression (lower part) analysis for predictors of arrhythmias, comparing patients with symptoms and those without symptoms. Overall clinical impact refers to the presence of one or more of the aforementioned clinical implications.
Abbreviations: CI, confidence interval; HR, hazard ratio; ICD, implantable cardioverter defibrillator; OR, odds ratio; PM, pacemaker; pts, patients; w, with; w/o, without.
Predictors of events
Analysing predictors of arrhythmic events, at univariate and multivariate Cox regression analysis (Table 3), the presence of symptoms before ILR implant (HR 2.5, 95% CI 1.3–4.9, P = .001) and age above 50 years (HR 1.7, 95% CI 1.1–2.5, P = .016) were found to be independently associated with the occurrence of arrhythmias during follow-up. The arrhythmic event distributions and clinical implications stratified according to the symptom status are presented in Table 2. Analysing predictors of VAs (Table 3), the only baseline variable related to the occurrence of VA, was VF inducibility during EPS (HR 9.0, 95% CI 3.4–24.2, P < .001).
Table 3.
Predictors of arrhythmic events in Brugada syndrome patients with implantable loop recorder
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |||
| Lower | Upper | Lower | Upper | |||||
| All arrhythmic events | ||||||||
| Female sex | 1.5 | 0.8 | 1.8 | .51 | ||||
| Age > 50 years | 1.8 | 1.2 | 2.8 | .002 | 1.7 | 1.1 | 2.5 | .016 |
| ILR for symptoms | 3.1 | 1.6 | 5.9 | .001 | 2.5 | 1.3 | 4.9 | .001 |
| ILR for syncope | 1.2 | 0.8 | 1.8 | .46 | ||||
| ILR for palpitations | 1.9 | 1.2 | 2.9 | .003 | 1.4 | 0.9 | 2.3 | .10 |
| Proband status | 1.6 | 0.9 | 2.7 | .069 | ||||
| Family history of BrS | 1.1 | 0.7 | 1.6 | .82 | ||||
| Family history of SCD | 1.2 | 0.8 | 1.9 | .37 | ||||
| Shanghai score | 1.0 | 0.9 | 1.2 | .65 | ||||
| Type 1 ECG | 1.0 | 0.7 | 1.5 | .92 | ||||
| SCN5A P/LP variant | 1.0 | 0.6 | 1.8 | .94 | ||||
| Positive EP study | 1.9 | 0.9 | 3.9 | .085 | ||||
| Ventricular arrhythmic events | ||||||||
| Female sex | 0.8 | 0.3 | 2.1 | .69 | ||||
| Age | 0.1 | 0.9 | 1.0 | .98 | ||||
| ILR for syncope | 1.3 | 0.5 | 3.3 | .51 | ||||
| ILR for symptoms | 32.1 | 0.5 | 2267.4 | .11 | ||||
| Proband status | 3.8 | 0.9 | 16.4 | .073 | ||||
| Family history of BrS | 1.0 | 0.4 | 2.7 | .95 | ||||
| Family history of SCD | 0.9 | 0.3 | 2.4 | .81 | ||||
| Shanghai score | 1.3 | 0.9 | 1.7 | .124 | ||||
| Type 1 ECG | 0.8 | 0.3 | 1.9 | .60 | ||||
| BrS pathogenic variant | 1.7 | 0.6 | 4.7 | .33 | ||||
| Positive EP study | 9.0 | 3.4 | 24.2 | <.001 | ||||
Univariate and multivariate COX regression analysis for predictors of arrhythmias.
Abbreviations: BrS, Brugada syndrome; CI, confidence interval; EP, electrophysiological; HR, hazard ratio; ICD, implantable cardioverter defibrillator; ILR, implantable loop recorder; OR, odds ratio; PM, pacemaker; SCD, sudden cardiac death.
Subgroups analysis
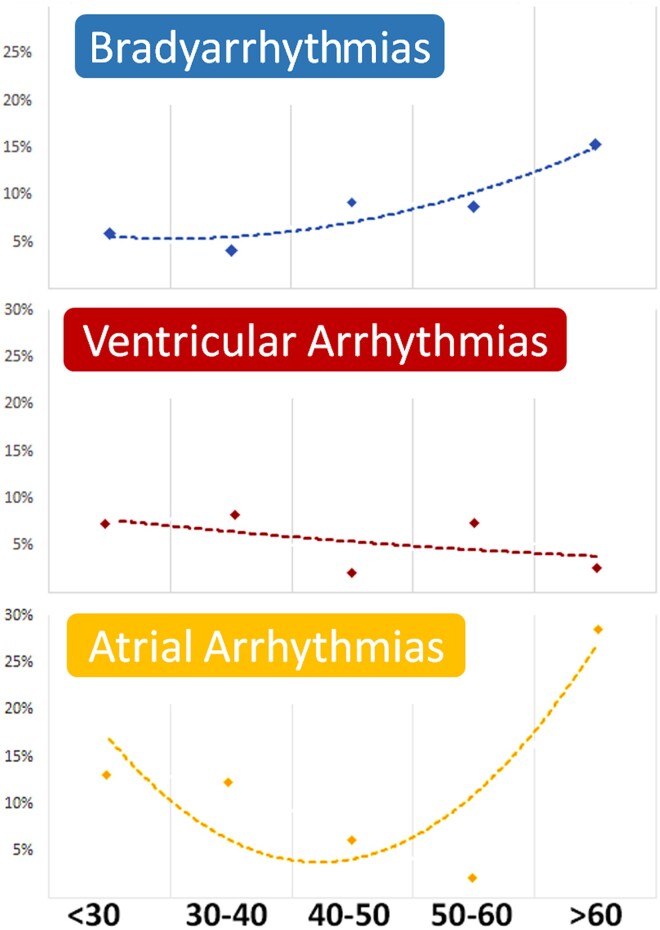
A sex-related sub-analysis is presented in Supplementary data online, Figure S1, showing no significant differences in the prevalence of different arrhythmias. The age-related sub-analysis is shown in Figure 4. The incidence of BAs progressively increased in the elderly, while a significant incidence of AF was present in both the elderly and younger adults. The incidence of VAs slowly decreased over time.
Figure 4.
Arrhythmia distribution in different age groups. In the bottom line, age groups divided by years of age are presented as < 30 years of age; 30–40 years of age, 40–50 years of age, 50–60 years of age, and > 60 years of age
Discussion
We report the largest cohort of patients with BrS and ILRs and expand the current knowledge on the value of continuous rhythm monitoring in BrS.
The main findings are: (i) ILR implantation in symptomatic patients with BrS allows to identify an arrhythmic event in nearly 30% of the patients, with significant clinical implications in 70% of them; (ii) the majority of detected arrhythmias are BAs or AAs, while the rate of sustained VAs is low; (iii) true arrhythmic syncope is infrequent in patients with unexplained syncope and mostly caused by BAs; (iv) symptom status can be used to guide ILR implantation.
Continuous rhythm monitoring in BrS: brady- and tachyarrhythmias
The 2018 ESC Guidelines on syncope and the 2022 guidelines on VAs consider the implant of an ILR in BrS patients with recurrent episodes of unexplained syncope.6,7 However, this recommendation is mostly based on expert opinion, as in the literature there is only small evidence supporting this approach.
Indeed, during the last decades, only few single-centre studies with small size patient populations have investigated the utility of ILRs in BrS patients.16 Giustetto et al. previously reported seven symptomatic patients with ILRs and BrS and observed no tachyarrhythmias during follow-up but one episode of 27-second pause leading to PM implantation.17 Few years later, the same group reported a larger cohort of 27 patients with unexplained syncope and ILR.18 In this group, the only recorded event was an asystolic pause of 24 s. Furthermore, Sakhi et al. observed that only 3 out of 20 BrS patients with ILR and syncope experienced NSVT, with no episode of SCD.19 Conversely, 11 patients (55%) had BAs leading to PM implantation, and one patient had AF.19 A larger series (50 patients) has recently been reported by Scrocco et al., with an arrhythmic event recorded in 11 patients (22%) during a median follow-up of 28 months (no sustained VA, 1 NSVT, 6 AF/PSVT, 4 BAs).10
Overall, all previous studies suggested two key points concerning symptomatic patients with BrS and ILRs: (i) arrhythmic syncope may result from both BAs and tachyarrhythmias; (ii) a high prevalence of arrhythmias, other than VAs, will likely be detected by ILR in symptomatic BrS patients.
Our data on a larger population of patients with BrS and ILRs confirm and strengthen the initially reported findings, showing a low prevalence of fatal arrhythmias in BrS patients without an ICD indication, with VAs being documented in a minority of patients (yearly incidence: 1.1%). Conversely, a non-negligible rate (25.4%) of non-VAs [mostly BAs (8.6%), and atrial tachyarrhythmias (15.7%)] were detected over a median follow-up of 3 years.
The prevalence of AF among BrS patients is known to be higher than in the general population but it has been characterized predominantly in the context of continuous monitoring of high-risk patients with ICDs, in whom AF occurrence is between 6% and 39%.20–23 Despite the presence of a specific ECG atrial phenotype displayed by BrS patients, the exact prevalence of AF among patients undergoing continuous rhythm monitoring has been largely unexplored until now.24 In our study, AF was documented by ILR in up to 10.8%. Moreover, in our cohort the yearly incidence of ventricular events was 2.18% in patients with syncope of arrhythmic or unknown origin. Based on previous studies on patients with syncope managed medically or with an ICD, the overall rate of ventricular events was comparable to our findings: 2.8 events per 100 person-years among patients with suspected arrhythmic syncope and 2.2 events per 100 person-years among patients with syncope of unknown cause.25 This is confirmed in another study reporting 2.6% events per years among patients with suspected cardiogenic syncope.4
Similarly, data on the prevalence of BAs has traditionally been limited to small case series, and bradycardia occurrence and need for pacing become often concealed after ICD implantation resulting in on-demand ventricular pacing.26 It has been previously reported that advanced AV block can be part of the clinical manifestation of BrS, being present in nearly 3% of patients. The prognostic value of advanced AV block remains controversial as well as the identification of the optimal strategy in these patients regarding device type selection (PM vs. ICD). On the other hand, it is well established the prognostic value of sinus node dysfunction in BrS, especially in the paediatric population.27,28 The prevalence of advanced AV block and sinus node dysfunction in our study population was 1.6% and 7.8%, respectively. Advanced AV block and sinus node dysfunction led to syncope in 68% and 64% of patients, respectively.
These findings importantly support the current guideline-based recommendation on the utility of ILR in BrS with respect to symptom correlation and arrhythmia detection.29
To date, no detailed information is available on the exact incidence of arrhythmias detected by ILR in young and otherwise healthy adults. Therefore, no specific comparison can be made between the burden of arrhythmias documented in our study and the arrhythmic burden of an age-related population of subjects without BrS. A previous study on a wearable monitoring device can be valuable in understanding the entity of the arrhythmic burden associated with BrS. The Apple Heart Study involved over 400 000 participants with a mean age of 41 years, undergoing continuous monitoring.30 An AF incidence of <0.5% was reported, whereas our study, in line with previous BrS literature, documented an AF incidence of >10%.
Predictors of arrhythmic events in BrS and ILRs
The ECG and clinical predictors of VAs in BrS patients have been widely assessed, with the spontaneous type 1 ECG and aborted SCD/VAs being the ones with the strongest association and leading to ICD implantation.1,7,31 In our study, VF induction at EPS was associated with recurrent arrhythmic ventricular events at follow-up. The value of EPS in BrS is controversial and its result should be taken into consideration based on the adopted protocol of ventricular stimulation and the overall risk profile of the patient.31–33
Moreover, symptom status before ILR implantation was found to be an independent predictor of overall arrhythmias. Indeed, our study results suggest that ILR implantation offers only modest benefits in asymptomatic patients, given the low rate of detected arrhythmias, and, therefore, its clinical value needs further evaluation. This is in line with a recent study from Gaita et al. that included only asymptomatic patients without ILR and documented a low ventricular arrhythmic event rate in this category of BrS patients.31 Conversely, in symptomatic (syncope or palpitations) patients that constitute the large majority of our patients’ cohort, the event rate is as high as 30%, and ILRs lead to significant clinical implications in 20%. This represents a significant step forward from the current guidelines, where only patients with syncope, and not those with palpitations, are considered appropriate candidates for ILR. Interestingly, although not surprisingly, the type of arrhythmias detected by ILR among patients with syncope and palpitations is different. Patients with syncope tend to experience more BAs and VAs, while those with palpitations are more likely to experience AAs.
Of note, the percentage of female patients in our study is slightly higher than what previously reported in other Brugada studies. This offers an important clinical input as it allows us to better characterize this usually underrepresented category. As shown in Supplementary data online, Figure S1, the occurrence of arrhythmic events is not substantially different in the three different arrhythmic categories.
Brugada syndrome and syncope
Arrhythmic syncope is considered a SCD prognostic marker in BrS.33,34 Therefore, a careful differentiation between probable arrhythmic syncope and non-arrhythmic syncope is of utmost importance.35 Different studies have shown that a history of arrhythmic syncope results in a two-times higher relative risk for VAs.35 Indeed, based on guidelines, in the presence of suspected arrhythmic syncope, an ICD should be implanted.5–7
However, two caveats should be considered. First, the predictive value of ‘suspected’ arrhythmic syncope is relatively low: only up to 15% of patients with arrhythmic syncope experience sustained VAs 9 years after ICD implantation.35 Secondly, the proportion of BrS patients with syncope unnecessarily treated with an ICD is not negligible, and inappropriate shocks can occur in up to 20% of cases.22,35 Moreover, the diagnosis of arrhythmic syncope mostly relies on patients’ memory and their ability to describe the event.36,37 This complexity is compounded by the fact that syncope is a frequent symptom: 30% of BrS patients will have experienced syncope at the time of diagnosis. The high prevalence of syncope, particularly vasovagal syncope, suggests that a patient with BrS may experience episodes of both vasovagal and arrhythmic syncopes.38 No single feature from medical history-taking is sufficient for differential diagnosis, and triggers or prodromes can be concurrent in neutrally mediated and arrhythmic syncope.8,25 Finally, BAs or atrial tachyarrhythmias can be the cause of the arrhythmic syncope, and, based on the current knowledge, patients with non-VAs may not need an ICD. Current guidelines suggest that instead of an ICD, an ILR should be considered in patients with recurrent episodes of unexplained syncope (class IIa, level of evidence C).6,7 Overall, our study supports and justifies the more conservative approach outlined in the guidelines, showing that VA rate among patients with syncope is low.
Limitations
Our study presents several limitations. Our cohort consists of a heterogeneous population with a low proportion of syncopal events suspected to be arrhythmic, thus limiting ability to perform a comprehensive analysis. Yet as stated before the ability to truly identify a syncopal event as arrhythmic is limited, making the inclusion of a significant portion of unselected syncopal cases a reflection of real-world clinical practices. The duration of our 3-year follow-up period is relatively brief, which could potentially result in underestimating event rates, especially considering the young age of our patient population. The rate of patients undergoing EPS (61%) may appear high. However, this rate is reflective of the presence of potential indicators of enhanced arrhythmic risk in our patient population, in which extensive efforts were made to exclude malignant VAs before ILR implantation.
Conclusions
ILR implantation identifies arrhythmic events in nearly 30% of symptomatic BrS patients and leads to significant clinical implications in 70% of them. The most commonly detected arrhythmias are AAs and BAs, while VAs are detected only in 7% of cases. Symptom status can be used to guide ILR implantation.
Supplementary Material
Acknowledgement
None.
Contributor Information
Marco Bergonti, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Via Tesserete 48, CH-6900 Lugano, Switzerland.
Frederic Sacher, Hôpital Cardiologique du Haut-Lévêque, CHU Bordeaux, L’Institut de Rythmologie et modélisation Cardiaque (LIRYC), Université Bordeaux, Bordeaux, France.
Elena Arbelo, Arrhythmia Section, Cardiology Department, Hospital Clinic, Universitat de Barcelona, Barcelona, Spain.
Lia Crotti, Center for Cardiac Arrhythmias of Genetic Origin and Laboratory of Cardiovascular Genetics, Istituto Auxologico Italiano, IRCCS, Milan, Italy; Departement of Medicine and Surgery, University Milano Bicocca, Milan, Italy.
Avi Sabbag, The Davidai Center for Rhythm Disturbances and Pacing, Chaim Sheba Medical Center, Tel Hashomer and the faculty of medicine, Tel-Aviv University, Tel-Aviv, Israel.
Michela Casella, Cardiology and Arrhythmology Clinic, University Hospital ‘Ospedali Riuniti’, Ancona, Italy; Department of Clinical, Special and Dental Sciences, Marche Polytechnic University, Ancona, Italy.
Johan Saenen, Department of Cardiology, University Hospital Antwerp, Edegem, Belgium.
Andrea Rossi, Arrhythmology Division, Fondazione Gabriele Monasterio CNR-Regione Toscana, via Giuseppe Moruzzi, Pisa, Italy.
Cinzia Monaco, Hôpital Cardiologique du Haut-Lévêque, CHU Bordeaux, L’Institut de Rythmologie et modélisation Cardiaque (LIRYC), Université Bordeaux, Bordeaux, France.
Luigi Pannone, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Brussels, Belgium.
Paolo Compagnucci, Cardiology and Arrhythmology Clinic, University Hospital ‘Ospedali Riuniti’, Ancona, Italy.
Vincenzo Russo, Cardiology Unit, Department of Translational Medical Sciences, University of Campania ‘Luigi Vanvitelli’, Monaldi Hospital, Naples, Italy.
Eyal Heller, The Davidai Center for Rhythm Disturbances and Pacing, Chaim Sheba Medical Center, Tel Hashomer and the faculty of medicine, Tel-Aviv University, Tel-Aviv, Israel.
Amato Santoro, Division of Cardiology, Cardio Thoracic and Vascular Department, Azienda Ospedaliera Universitaria Senese, Siena, Italy.
Paola Berne, Department of Cardiology, Ospedale Santissima Annunziata, University of Sassari, Sassari, Italy.
Antonio Bisignani, Institute of Cardiology, Catholic University of the Sacred Heart, Roma, Italy.
Enrico Baldi, Arrhythmia and Electrophysiology, Division of Cardiology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Olivier Van Leuven, Department of Cardiology, University Hospital Antwerp, Edegem, Belgium.
Federico Migliore, Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padova, Padua, Italy.
Lorenzo Marcon, Department of Clinical Electrophysiology & Cardiac Pacing, Centro Cardiologico Monzino IRCCS, Milan, Italy; Department of Biomedical, Surgery and Dentist Sciences, University of Milan, Milan, Italy.
Federica Dagradi, Center for Cardiac Arrhythmias of Genetic Origin and Laboratory of Cardiovascular Genetics, Istituto Auxologico Italiano, IRCCS, Milan, Italy.
Irene Sfondrini, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Via Tesserete 48, CH-6900 Lugano, Switzerland.
Federico Landra, Arrhythmology Division, Fondazione Gabriele Monasterio CNR-Regione Toscana, via Giuseppe Moruzzi, Pisa, Italy; Division of Cardiology, Cardio Thoracic and Vascular Department, Azienda Ospedaliera Universitaria Senese, Siena, Italy.
Angelo Comune, Cardiology Unit, Department of Translational Medical Sciences, University of Campania ‘Luigi Vanvitelli’, Monaldi Hospital, Naples, Italy.
María Cespón-Fernández, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Brussels, Belgium.
Martina Nesti, Arrhythmology Division, Fondazione Gabriele Monasterio CNR-Regione Toscana, via Giuseppe Moruzzi, Pisa, Italy.
Francesco Santoro, Cardiothoracic Department, Cardiology Unit, Policlinico Riuniti, Foggia, Italy.
Michele Magnocavallo, Institute of Cardiology, Catholic University of the Sacred Heart, Roma, Italy.
Alessandro Vicentini, Arrhythmia and Electrophysiology, Division of Cardiology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Sergio Conti, Department of Cardiac Electrophysiology, ARNAS Ospedali Civico Di Cristina Benfratelli, Palermo, Italy.
Valentina Ribatti, Department of Clinical Electrophysiology & Cardiac Pacing, Centro Cardiologico Monzino IRCCS, Milan, Italy; Department of Biomedical, Surgery and Dentist Sciences, University of Milan, Milan, Italy.
Pedro Brugada, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Brussels, Belgium.
Carlo de Asmundis, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Brussels, Belgium.
Josep Brugada, Arrhythmia Section, Cardiology Department, Hospital Clinic, Universitat de Barcelona, Barcelona, Spain.
Claudio Tondo, Department of Clinical Electrophysiology & Cardiac Pacing, Centro Cardiologico Monzino IRCCS, Milan, Italy; Department of Biomedical, Surgery and Dentist Sciences, University of Milan, Milan, Italy.
Peter J Schwartz, Center for Cardiac Arrhythmias of Genetic Origin and Laboratory of Cardiovascular Genetics, Istituto Auxologico Italiano, IRCCS, Milan, Italy.
Michel Haissaguerre, Hôpital Cardiologique du Haut-Lévêque, CHU Bordeaux, L’Institut de Rythmologie et modélisation Cardiaque (LIRYC), Université Bordeaux, Bordeaux, France.
Angelo Auricchio, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Via Tesserete 48, CH-6900 Lugano, Switzerland; Faculty of Biomedical Sciences, Università della Svizzera Italiana, via la Santa 1, 6962 Lugano, Switzerland.
Giulio Conte, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Via Tesserete 48, CH-6900 Lugano, Switzerland; Faculty of Biomedical Sciences, Università della Svizzera Italiana, via la Santa 1, 6962 Lugano, Switzerland.
Supplementary Data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
F.S. received speaking honorarium and/or consulting fees from Abbott, Biosense Webster, Boston Scientific, Inheart, Medtronic, and Microport. M.C. received speaker fees from Abbott Medical and Biosense Webster. C.T. serves as Member of Advisory Board of Medtronic, Boston Scientific. He receives lecture and grant travel fees from Medtronic, Abbott Medical, Boston Scientific, and Atricure. P.B. received compensation for teaching purposes from Biotronik. C.D.A. receives research grants on behalf of the centre from Biotronik, Medtronic, Abbott, LivaNova, Boston Scientific, AtriCure, Philips, and Acutus; C.D.A. received compensation for teaching purposes and proctoring from Medtronic, Abbott, Biotronik, Livanova, Boston Scientific, Atricure, Acutus Medical Daiichi Sankyo. A.A. is a consultant to Boston Scientific, Cairdac, Corvia, Microport CRM, EPD Philips, and Radcliffe Publisher. He received speaker fees from Boston Scientific, Medtronic, and Microport. He participates in clinical trials sponsored by Boston Scientific, Medtronic, EPD-Philips. He has intellectual properties with Boston Scientific, Biosense Webster, and Microport CRM. G.C. has received a research grant (PZ00P3_180055) from the Swiss National Science Foundation (SNSF). All other co-authors do not report conflict of interest.
Data Availability
The datasets generated and/or analysed during the current study are not publicly available to maintain patient confidentiality but are available from the corresponding author on reasonable request and after the agreement of all the co-authors.
Funding
This study was fully supported by a research grant from the Swiss National Science Foundation (SNSF) (PZ00P3_180055). L.C., F.D., and P.J.S. have been supported by the Italian Ministry of Health.
Ethical Approval
The study was approved by the local ethics committees (Swiss Ethics, approval number: BASEC 2019-00754/CE 3476) and data protection agencies and conforms to the Declaration of Helsinki
Pre-registered Clinical Trial Number
The pre-registered clinical trial number is: not applicable. It was a retrospective registry.
References
- 1. Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 2013;10:1932–63. 10.1016/j.hrthm.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 2. Sacher F, Arsac F, Wilton SB, Derval N, Denis A, de Guillebon M, et al. Syncope in Brugada syndrome patients: prevalence, characteristics, and outcome. Heart Rhythm 2012;9:1272–9. 10.1016/j.hrthm.2012.04.013 [DOI] [PubMed] [Google Scholar]
- 3. Sieira J, Ciconte G, Conte G, de Asmundis C, Chierchia G-B, Baltogiannis G, et al. Long-term prognosis of drug-induced Brugada syndrome. Heart Rhythm 2017;14:1427–33. 10.1016/j.hrthm.2017.04.044 [DOI] [PubMed] [Google Scholar]
- 4. Hernandez-Ojeda J, Arbelo E, Jorda P, Borras R, Campuzano O, Sarquella-Brugada G, et al. The role of clinical assessment and electrophysiology study in Brugada syndrome patients with syncope. Am Heart J 2020;220:213–23. 10.1016/j.ahj.2019.10.016 [DOI] [PubMed] [Google Scholar]
- 5. Antzelevitch C, Yan G-X, Ackerman MJ, Borggrefe M, Corrado D, Guo J, et al. J-Wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. Heart Rhythm 2016;13:e295–324. 10.1016/j.hrthm.2016.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brignole M, Moya A, de Lange FJ, Deharo J-C, Elliott PM, Fanciulli A, et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J 2018;39:1883–948. 10.1093/eurheartj/ehy037 [DOI] [PubMed] [Google Scholar]
- 7. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. 10.1093/eurheartj/ehac262 [DOI] [PubMed] [Google Scholar]
- 8. Mascia G, Della Bona R, Ameri P, Canepa M, Porto I, Parati G, et al. Brugada syndrome and syncope: a practical approach for diagnosis and treatment. Europace 2021;23:996–1002. 10.1093/europace/euaa370 [DOI] [PubMed] [Google Scholar]
- 9. Jespersen CHB, Krøll J, Bhardwaj P, Winkel BG, Jacobsen PK, Jøns C, et al. Severity of Brugada syndrome disease manifestation and risk of new-onset depression or anxiety: a Danish nationwide study. Europace 2023;25:euad112. 10.1093/europace/euad112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scrocco C, Ben-Haim Y, Devine B, Tome-Esteban M, Papadakis M, Sharma S, et al. Role of subcutaneous implantable loop recorder for the diagnosis of arrhythmias in Brugada syndrome: a United Kingdom single-center experience. Heart Rhythm 2022;19:70–8. 10.1016/j.hrthm.2021.08.034 [DOI] [PubMed] [Google Scholar]
- 11. Kubala M, Aïssou L, Traullé S, Gugenheim A-L, Hermida J-S. Use of implantable loop recorders in patients with Brugada syndrome and suspected risk of ventricular arrhythmia. Europace 2012;14:898–902. 10.1093/europace/eur319 [DOI] [PubMed] [Google Scholar]
- 12. Milman A, Sabbag A, Conte G, Postema PG, Andorin A, Gourraud J-B, et al. Characteristics of patients with spontaneous versus drug-induced Brugada electrocardiogram: sub-analysis from the SABRUS. Circ Arrhythm Electrophysiol 2023;16:e011360. 10.1161/CIRCEP.122.011360 [DOI] [PubMed] [Google Scholar]
- 13. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42:3427–520. 10.1093/eurheartj/ehab364 [DOI] [PubMed] [Google Scholar]
- 14. Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri N, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. J Arrhythm 2019;35:323–484. 10.1002/joa3.12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomström-Lundqvist C, et al. 2019 ESC guidelines for the management of patients with supraventricular tachycardia. Eur Heart J 2020;41:655–720. 10.1093/eurheartj/ehz467 [DOI] [PubMed] [Google Scholar]
- 16. Champagne J, Philippon F, Gilbert M, Molin F, Blier L, Nault I, et al. The Brugada syndrome in Canada: a unique French-Canadian experience. Can J Cardiol 2007;23:71B–5B. 10.1016/S0828-282X(07)71014-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giustetto C, Drago S, Demarchi PG, Dalmasso P, Bianchi F, Masi AS, et al. Risk stratification of the patients with Brugada type electrocardiogram: a community-based prospective study. Europace 2009;11:507–13. 10.1093/europace/eup006 [DOI] [PubMed] [Google Scholar]
- 18. Giustetto C, Cerrato N, Ruffino E, Gribaudo E, Scrocco C, Barbonaglia L, et al. Etiological diagnosis, prognostic significance and role of electrophysiological study in patients with Brugada ECG and syncope. Int J Cardiol 2017;241:188–93. 10.1016/j.ijcard.2017.03.019 [DOI] [PubMed] [Google Scholar]
- 19. Sakhi R, Assaf A, Theuns DAMJ, Verhagen JMA, Szili-Torok T, Roos-Hesselink JW, et al. Outcome of insertable cardiac monitors in symptomatic patients with Brugada syndrome at low risk of sudden cardiac death. Cardiology 2020;145:413–20. 10.1159/000507075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tijskens M, Bergonti M, Spera F, Ascione C, Saenen J, Huybrechts W, et al. Etiology and outcome of catheter ablation in patients with onset of atrial fibrillation <45 years of age. Am J Cardiol 2022;166:45–52. 10.1016/j.amjcard.2021.11.030 [DOI] [PubMed] [Google Scholar]
- 21. Conte G, Chierchia G-B, Wauters K, De Asmundis C, Sarkozy A, Levinstein M, et al. Pulmonary vein isolation in patients with Brugada syndrome and atrial fibrillation: a 2-year follow-up. Europace 2014;16:528–32. 10.1093/europace/eut309 [DOI] [PubMed] [Google Scholar]
- 22. Kusano KF, Taniyama M, Nakamura K, Miura D, Banba K, Nagase S, et al. Atrial fibrillation in patients with Brugada syndrome relationships of gene mutation, electrophysiology, and clinical backgrounds. J Am Coll Cardiol 2008;51:1169–75. 10.1016/j.jacc.2007.10.060 [DOI] [PubMed] [Google Scholar]
- 23. Conte G, Caputo ML, Volders PGA, Luca A, Mainardi L, Schotten U, et al. Concealed abnormal atrial phenotype in patients with Brugada syndrome and no history of atrial fibrillation. Int J Cardiol 2018;253:66–70. 10.1016/j.ijcard.2017.09.214 [DOI] [PubMed] [Google Scholar]
- 24. Zanchi B, Faraci FD, Gharaviri A, Bergonti M, Monga T, Auricchio A, et al. Identification of Brugada syndrome based on P-wave features: an artificial intelligence-based approach. Europace 2023;25:euad334. 10.1093/europace/euad334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mascia G, Della Bona R, Ameri P, Canepa M, Porto I, Brignole M, et al. Brugada syndrome and syncope: a systematic review. J Cardiovasc Electrophysiol 2020;31:3334–8. 10.1111/jce.14787 [DOI] [PubMed] [Google Scholar]
- 26. Kamakura T, Sacher F, Katayama K, Ueda N, Nakajima K, Wada M, et al. High-risk atrioventricular block in Brugada syndrome patients with a history of syncope. J Cardiovasc Electrophysiol 2021;32:772–81. 10.1111/jce.14876 [DOI] [PubMed] [Google Scholar]
- 27. Sieira J, Conte G, Ciconte G, Chierchia G-B, Casado-Arroyo R, Baltogiannis G, et al. A score model to predict risk of events in patients with Brugada syndrome. Eur Heart J 2017;38:1756–63. 10.1093/eurheartj/ehx119 [DOI] [PubMed] [Google Scholar]
- 28. Conte G, Dewals W, Sieira J, de Asmundis C, Ciconte G, Chierchia G-B, et al. Drug-induced Brugada syndrome in children: clinical features, device-based management, and long-term follow-up. J Am Coll Cardiol 2014;63:2272–9. 29. 10.1016/j.jacc.2014.02.574 [DOI] [PubMed] [Google Scholar]
- 29. Conte G, DE Asmundis C, Sieira J, Levinstein M, Chierchia G-B, DI Giovanni G, et al. Clinical characteristics, management, and prognosis of elderly patients with Brugada syndrome. J Cardiovasc Electrophysiol 2014;25:514–9. 10.1111/jce.12359 [DOI] [PubMed] [Google Scholar]
- 30. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–17. 10.1056/NEJMoa1901183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gaita F, Cerrato N, Giustetto C, Martino A, Bergamasco L, Millesimo M, et al. Asymptomatic patients with Brugada ECG pattern: long-term prognosis from a large prospective study. Circulation 2023;148:1543–55. 10.1161/CIRCULATIONAHA.123.064689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Conte G, Sieira J, Ciconte G, de Asmundis C, Chierchia G-B, Baltogiannis G, et al. Implantable cardioverter-defibrillator therapy in Brugada syndrome: a 20-year single-center experience. J Am Coll Cardiol 2015;65:879–88. 10.1016/j.jacc.2014.12.031 [DOI] [PubMed] [Google Scholar]
- 33. Sacher F, Probst V, Iesaka Y, Jacon P, Laborderie J, Mizon-Gérard F, et al. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study. Circulation 2006;114:2317–24. 10.1161/CIRCULATIONAHA.106.628537 [DOI] [PubMed] [Google Scholar]
- 34. Probst V, Veltmann C, Eckardt L, Meregalli PG, Gaita F, Tan HL. Long-term prognosis of patients diagnosed with Brugada syndrome: results from the FINGER Brugada syndrome registry. Circulation 2010;121:635–43. 10.1161/CIRCULATIONAHA.109.887026 [DOI] [PubMed] [Google Scholar]
- 35. Olde Nordkamp LRA, Postema PG, Knops RE, van Dijk N, Limpens J, Wilde AA, et al. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: a systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm 2016;13:443–54. 10.1016/j.hrthm.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 36. Mascia G, Crotti L, Groppelli A, Canepa M, Merlo AC, Benenati S, et al. Syncope in hypertrophic cardiomyopathy (part I): an updated systematic review and meta-analysis. Int J Cardiol 2022;357:88–94. 10.1016/j.ijcard.2022.03.028 [DOI] [PubMed] [Google Scholar]
- 37. Brignole M, Cecchi F, Anastasakis A, Crotti L, Deharo JC, Elliott PM, et al. Syncope in hypertrophic cardiomyopathy (part II): an expert consensus statement on the diagnosis and management. Int J Cardiol 2023;370:330–7. 10.1016/j.ijcard.2022.10.153 [DOI] [PubMed] [Google Scholar]
- 38. Hamilton G, O’Donnell D, Han H-C. Brugada syndrome and undifferentiated syncope: use of an implantable loop recorder to document causation. Med J Aust 2018;209:113–4. 10.5694/mja17.01117 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available to maintain patient confidentiality but are available from the corresponding author on reasonable request and after the agreement of all the co-authors.