Table 3.
Asymmetric Henry reactions of various aldehydes with nitromethane catalysed by copper(II) complexes of ligands IIIa and IIIb.
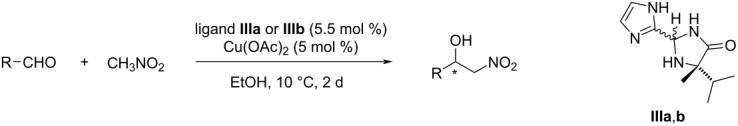
| ||||
|
| ||||
| Aldehyde | Ligand IIIa (trans) |
Ligand IIIb (cis) |
||
| R | Conva [%] | eeb [%] | Conva [%] | eeb [%] |
|
| ||||
| Ph | 63 | 89 (R) | 57 | 86 (S) |
| 4-NO2C6H4 | 97 | 84 (R) | 89 | 76 (S) |
| 2-CH3OC6H4 | 98 | 94 (R) | 89 | 93 (S) |
| 4-ClC6H4 | 82 | 89 (R) | 76 | 85 (S) |
| 2-thienyl | 40 | 88 (R) | 36 | 80 (S) |
| naphth-2-yl | 63 | 89 (R) | 63 | 84 (S) |
| PhCH2CH2 | 45 | 89 (R) | 44 | 74 (S) |
| t-Bu | 62 | 96 (R) | 49 | 92 (S) |
| iPr | 90 | 94 (R) | 64 | 88 (S) |
aThe conversion was determined by 1H NMR analysis of the crude product. bThe enantiomeric excess was determined by chiral HPLC.
