Figure 2. Cancer Drugs Granted Accelerated Approval From January 2013 to May 2017.
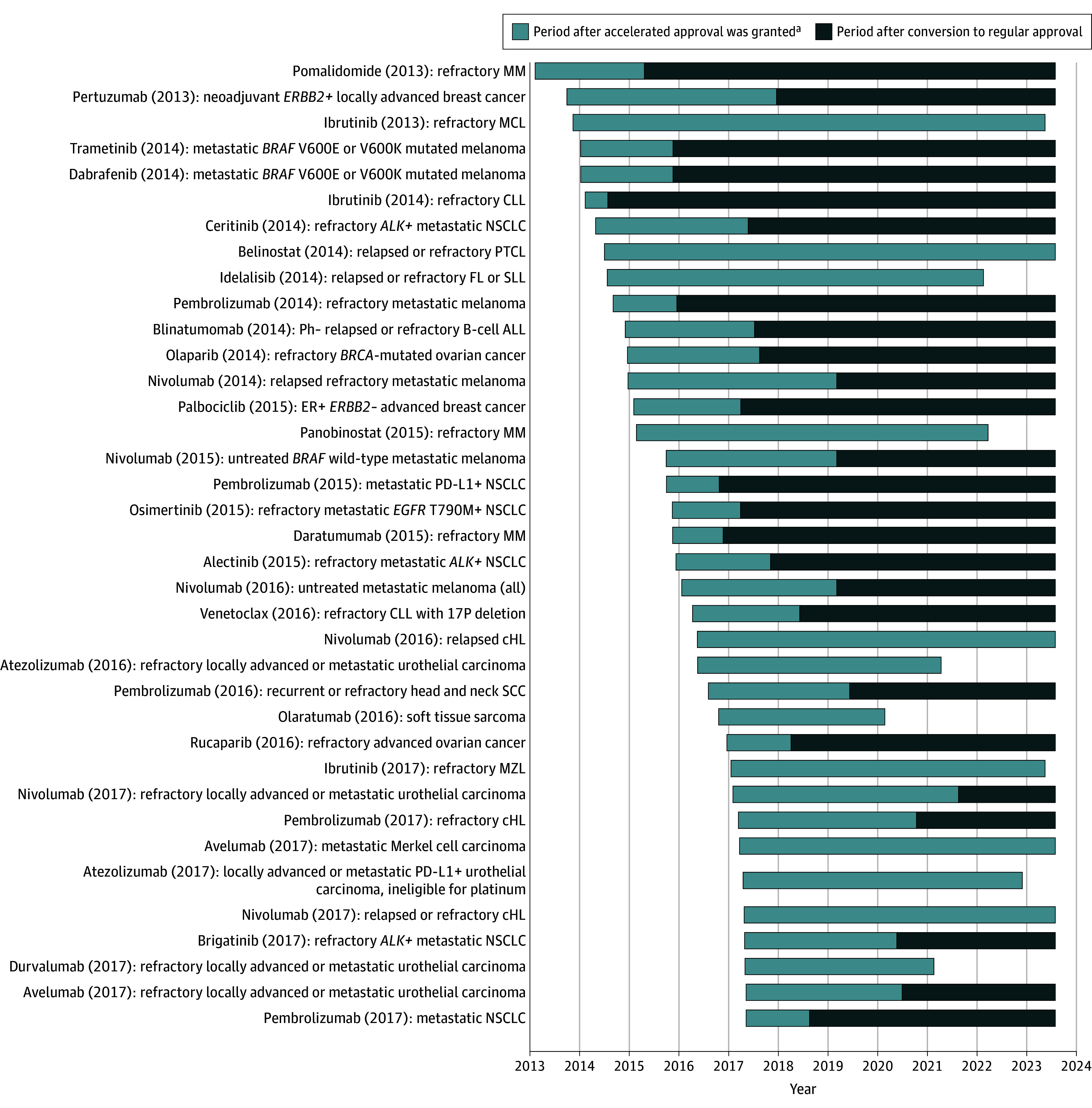
This figure excludes ongoing accelerated approvals with less than 5 years of follow-up (n = 74). Drug (year of accelerated approval) indicates accelerated approval indication. ALL indicates acute lymphoblastic leukemia; cHL, classical Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; ER, estrogen receptor; FL, follicular lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; MZL, marginal zone lymphoma; NSCLC, non–small cell lung cancer; PD-L1, programmed cell death ligand 1; PTCL, peripheral T-cell lymphoma; SCC, squamous cell carcinoma; and SLL, small lymphocytic lymphoma.
aWithdrawn drugs are represented by the lighter bars ending prior to the cutoff date of August 1, 2023.
