Figure 3. Cancer Drugs Granted Accelerated Approval Since 2017 .
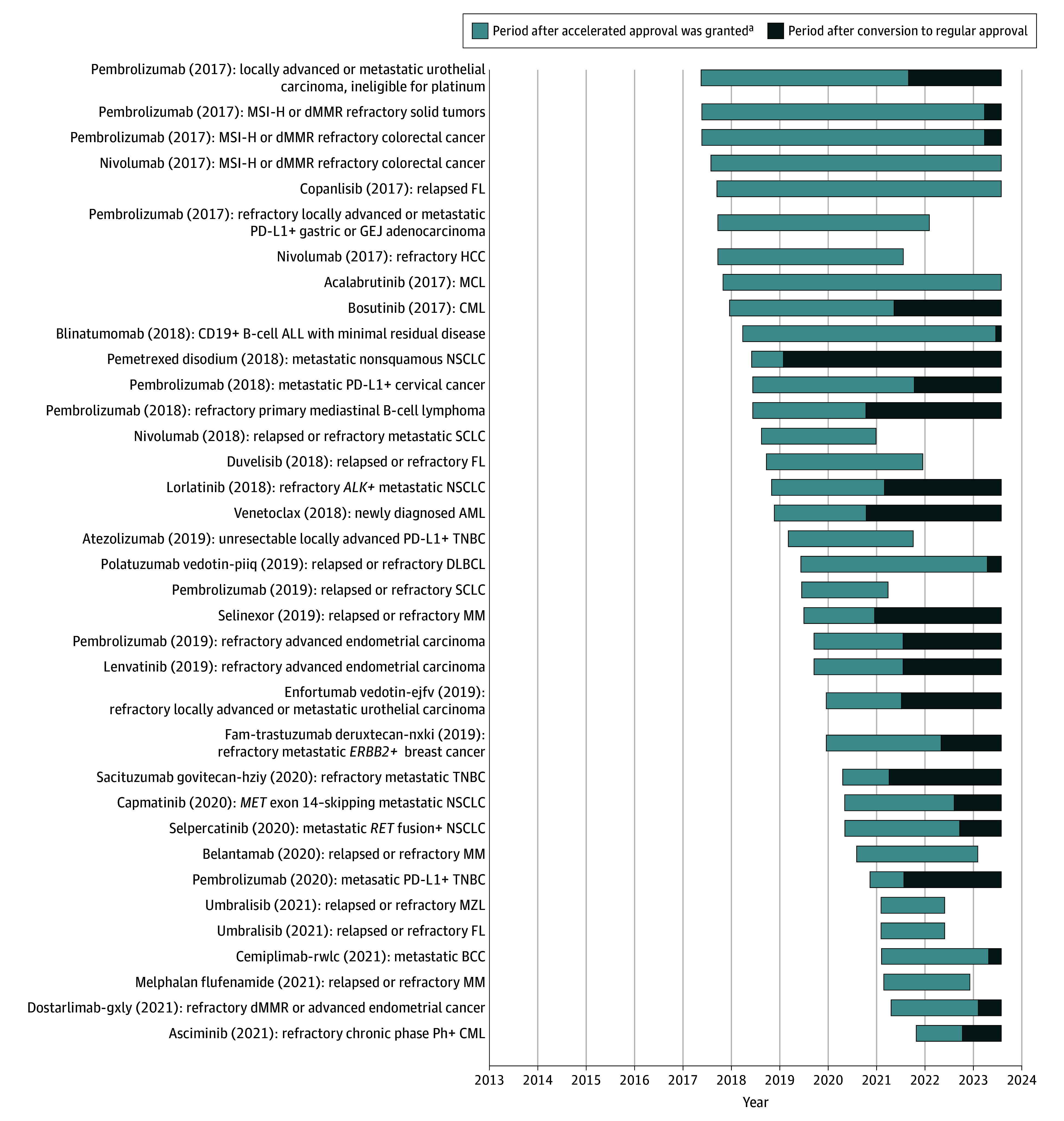
This figure excludes ongoing accelerated approvals with less than 5 years of follow-up (n = 74). Drug (year of accelerated approval) indicates accelerated approval indication. ALL indicates acute lymphoblastic leukemia; AML, acute myeloid leukemia; BCC, basal cell carcinoma; CML, chronic myeloid leukemia; DLBCL, diffuse large B-cell lymphoma; dMMR, mismatch repair deficient; FL, follicular lymphoma; GEJ, gastroesophageal junction; HCC, hepatocellular carcinoma; MCL, mantle cell lymphoma; MM, multiple myeloma; MSI-H, microsatellite instability high; MZL, marginal zone lymphoma; NSCLC, non–small cell lung cancer; PD-L1, programmed cell death ligand 1; SCLC, small cell lung cancer; and TNBC, triple-negative breast cancer.
aWithdrawn drugs are represented by the lighter bars ending prior to the cutoff date of August 1, 2023.
