Abstract
Rhipicephalus microplus ticks feed on a bovine host for three weeks. At the attachment site, inflammatory and immune responses are triggered resulting in the recruitment of cells and production of a set of immunological mediators. To oppose the host’s immune responses, ticks inoculate bioactive salivary molecules capable of interfering with these defense mechanisms. Serpins are among the most frequent molecules present in tick saliva and have been shown to negatively affect the host’s anti-tick immunity. R. microplus has at least eighteen full-length serpins (RmS) and eleven are transcribed during blood feeding. Among them, RmS-3, RmS-6, and RmS-17 are present in the saliva of engorged females. Here, the effect of these serpins on the immune responses was evaluated in cells involved in innate/inflammatory (mast cells and macrophages) and adaptive (T cells) immunity. RmS-3 modulated mast cells due to its inhibitory activity on peritoneal rat chymase and on vascular permeability in acute inflammation. In addition, both RmS-6 and RmS-17 inhibited vascular permeability. Of the three serpins studied, neither affected activation nor inflammatory cytokine production by murine macrophages. On the other hand, RmS-3 and RmS-17 presented an inhibitory effect on the metabolic activity of lymphocytes, with the latter being the most potent, while RmS-6 had no effect on it. This activity was associated with a decrease in lymphocyte proliferation, but not with induction of cell death. The present study highlights the powerful modulatory role of tick salivary serpins in the host’s immune system and inspire the discovery of targets for the treatment of inflammatory/immune disorders.
Keywords: Serpins, saliva, Immunomodulation, Mast Cells, Lymphocytes
1. Introduction
Rhipicephalus microplus, the cattle tick, has a major effect on livestock husbandry especially in tropical countries. Along with anemia, weight loss and decreased milk production caused by the large blood intake during heavy infestations, this species also acts as a vector of tick-borne pathogens such as the etiologic agents of babesiosis and anaplasmosis (Grisi et al., 2014; Jongejan and Uilenberg, 2004). To gain access to host blood, R. microplus lacerates the skin creating a pool of blood surrounding the lesion were the tick feeds for up to three weeks, resulting in the activation of the host’s defenses such as pain, coagulation, inflammation, adaptive immune responses and wound healing (Francischetti et al., 2009; Heinze et al., 2012a, 2012b). Meanwhile, the tick inoculates saliva – a complex mixture of protein and non-protein bioactive molecules – at the feeding site to counterbalance the host responses. Rhipicephalus microplus sialotranscriptomes revealed the presence of transcripts coding for Kunitz-type, serpins, cystatins, lipocalins, and many other secreted protein families with modulatory potential on their hosts (Chmelař et al., 2017; Maruyama et al., 2017; Tirloni et al., 2014a, 2014b); however, the biological effects of these molecules on the vertebrate immune system are largely unknown.
Serpins are part of a ubiquitous superfamily whose many members are protease inhibitors, although some of them evolved distinct functions such as storage, transport, blood pressure regulation and molecular chaperoning, among others (Gettins, 2002). Serpins have been described to interfere in several immune functions of arthropods such as regulation of the Toll pathway and inhibition of the prophenoloxidase, which directly influences the synthesis of melanin (Meekins et al., 2017). Salivary serpins can also participate in host-parasite interactions by being secreted and injected into the host’s skin during the blood meal (Kim et al., 2015; Tirloni et al., 2016). Therefore, serpins present in the saliva of blood feeding arthropods can interfere with the host hemostasis, kallikrein-kinin system, and immune responses, thus allowing the parasite to evade the host defenses at the feeding site. Several authors have reported and characterized serpins in the saliva of different tick species (Chmelař et al., 2017; Meekins et al., 2017; Parizi et al., 2018). A few of these serpins have been described to act in the modulation of the host’s immune system such as Ixodes persulcatus Ipis-1 (Toyomane et al., 2016), Ixodes ricinus immunosuppressor (Iris) and I. ricinus serpin-2 (IRS-2) (Chmelar et al., 2011; Páleníková et al., 2015), Rhipicephalus haemaphysaloides RHS2 (Xu et al., 2019), Amblyomma americanum AAS27 (Tirloni et al., 2019), and Haemaphysalis longicornis HlSerpin-a and HlSerpin-b (Wang et al., 2019).
Rhipicephalus microplus putative serpin coding sequences have been identified (Rodriguez-Valle et al., 2015; Tirloni et al., 2014b). Four of these serpins possess known biochemical functions: RmS-3 inhibits chymase, chymotrypsin, and elastase; RmS-6 inhibits trypsin, coagulation factors such as Xa, factor XIa and plasmin (Tirloni et al., 2016); RmS-15 inhibits thrombin (Xu et al., 2016); RmS-17 inhibits chymotrypsin, factor XIa, trypsin and plasmin, and delays plasma clotting time (Tirloni et al., 2016). Both RmS-3 and RmS-17 present inhibitory activity against cathepsin G (Tirloni et al., 2016). Considering the inhibition profile exhibited by RmS-3, RmS-6 and RmS-17 and their potential role as immunomodulators, the present study was designed to investigate the effect of these serpins on the biology of cells involved in inflammation and adaptive immune responses to ticks, such as mast cells, macrophages and lymphocytes.
2. Material and methods
2.1. Animals
Male Wistar rats, 6–8-week-old, were supplied by the Central Animal Facility, Institute of Basic Health Sciences, Universidade Federal do Rio Grande do Sul (UFRGS). Female C57BL/6 mice, 4–6-week-old, were bred and maintained at the Isogenic Breeding Unit of the Department of Immunology, Instituto de Ciências Biomédicas, Universidade de São Paulo (ICB/USP). Female BALB/c mice, 4–6-week-old, were originally purchased from CEMIB/UNICAMP (Campinas, SP, Brazil) and bred at UFRGS. During all manipulation procedures, animals were maintained under specific pathogen-free conditions and kept under controlled temperature and luminosity, with food and water ad libitum. All procedures involving vertebrate animals were carried out in accordance with internationally recognized guidelines and in agreement with the Brazilian National Law number 11,794, Decree 6,899 and the Normative Resolutions of the National Council for the Control of Animal Experimentation (CONCEA). The procedures were approved by the Institutional Animal Care and Use Committee from the UFRGS (protocols # 28371 and 30927) and from ICB/USP (protocol # 55/2015).
2.2. Expression and purification of rRmS-3, rRmS-6 and rRmS-17
Recombinant RmS-3, RmS-6 and RmS-17 were expressed and purified as previously described (Tirloni et al., 2016). Affinity-purified proteins were dialyzed against 20 mM Tris-HCl, NaCl 150 mM buffer pH 7.4, and stored at −80 °C until use. Endotoxin was removed using the high capacity endotoxin removal spin columns kit and endotoxin level was estimated using Limulus amebocyte lysate assay (Thermo Fischer Scientific, Waltham, MA, USA), following the manufacturer’s instructions. Endotoxin contamination did not exceed 11 EU/mL in any of the protein samples used for the following assays.
2.3. Miles assay for vascular permeability
Wistar rats were anesthetized by an intraperitoneal injection of xylazin (10 mg/kg) and ketamine (75 mg/kg) and injected intravenously with 700 μL of Evans blue dye (50 mg/kg in saline) through the tail vein. After 5 minutes, animals were intradermally injected on the dorsal region (100 μL - final volume) with: (i) saline, (ii) RmS proteins (25 μg) in saline, (iii) agonist in saline, and (iv) agonist plus RmS (25 μg) in saline. Agonists used for Miles assay were formalin 2% for RmS-6 and RmS-17 (Cattaruzza et al., 2014), and compound 48/80 (1 μg per spot) for RmS-3 (Chatterjea et al., 2012). Two spots of each treatment were performed per animal (n = 6 per protein). After 60 minutes, animals were euthanized and an area of skin that included the entire injection sites was carefully removed and photographed. Evans blue dye spots on skin were excised and the dye was extracted incubating skin with 2.5 mL of formamide 50% for 24 hours at 55 °C. After centrifugation at 1,500 × g for 10 minutes, absorbance of the supernatant was measured at OD620nm (Müller et al., 2009).
2.4. Enzymatic assays
For rat peritoneum-derived cell chymase activity assay, Wistar male rats (n = 3) were euthanized and the peritoneal cavity was washed with 10 mL of cold sterile phosphate-buffered saline (PBS), pH 7.4. The cell-free supernatant was discarded after centrifugation (300 × g for 5 min at 4 °C) and the cells in the pellet were suspended in red blood cell lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.4), incubated for 5 minutes at room temperature following centrifugation (300 × g for 5 min at 4 °C). Supernatant was discarded and washed repeated twice. Cell pellet was suspended in lysis buffer (20 mM Tris-HCl, 2 M NaCl, pH 7.4). After lysis, extract was centrifuged (12,000 g for 15 min at 4 °C). Supernatant containing chymase activity (1 U) was incubated with recombinant serpins (1 μM) at 37 °C for 15 min. Reaction was triggered following addition of substrate N-Succinyl-Ala-Ala-Pro-Phe-pNA (0.2 mM - final concentration). Protease kinetics was monitored for 15 min at 30 °C with reads at every 11 seconds in triplicate using a SpectraMax M3 plate reader (Molecular Devices, San José, CA, USA). One unit of chymase is defined as the amount of protease necessary to achieve a velocity of 0.020 mOD405nm/s using experimental conditions described above.
2.5. Macrophage cultures
Peritoneal macrophages were recruited by intraperitoneal injection of mice with 1 mL 4% sterile thioglycolate medium (Becton, Dickinson and Company, Sparks, MD, USA). After 4 days, the animals were euthanized, and the peritoneal cavity lavage was collected with 5 mL of cold sterile PBS (pH 7.4). The cell-free supernatant was discarded after centrifugation (300 × g for 5 min at 4 °C) and the cells in the pellet were suspended in RPMI 1640 medium (Gibco Invitrogen, Grand Island, NY, USA), diluted in Turk’s solution (4 mg/L gentian violet in 3% acetic acid) and counted in a Neubauer chamber. A suspension containing 2 × 106 cells/mL was prepared in RPMI 1640 medium, distributed into sterile 96-well plates in aliquots of 100 μL/well and incubated for 2 h at 37 °C and 5% CO2 for macrophage adhesion. Cell monolayers were washed 3 times with warm PBS (at 37 °C) to remove non-adherent cells and the adherent cells (considered macrophages) were cultured in complete medium [RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, 25 mM HEPES and 2.5 × 105 M 2-mercaptoethanol (all from Gibco Invitrogen)] as described (Breijo et al., 2018).
2.6. Nitric oxide (NO) determination
Macrophages were prepared as described earlier and maintained in complete medium (control group) or pre-incubated with R. microplus serpins (10–1000nM) for 1 h followed by activation with 10 ng/mL of ultrapure LPS (InvivoGen, San Diego, CA, USA) plus 10 ng/mL of murine IFN-γ (Sigma-Aldrich, St. Louis, MO, USA). Cell-free supernatant was collected after 48 h and nitrite (NO2−) was evaluated in the culture supernatant by Griess reaction as previously described (Medeiros et al., 2004; Sá-Nunes et al., 2007a). Briefly, equal volumes of the supernatants and the Griess reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% N-(1-Naphthyl) ethylenediamine dihydrochloride in distilled water, v/v) were mixed and incubated for 10 min at room temperature. The optical density of each well was evaluated at OD554nm on a plate reader (SpectraMax M3, Molecular Devices, San José, CA, USA) and NO2- concentrations were deduced from a standard curve prepared with sodium nitrite (NaNO2) concentrations dissolved in complete medium.
2.7. Phagocytosis assay
Peritoneal macrophages were collected as described earlier. A suspension of 1 × 106 cells/mL was distributed in 24-well plates containing a glass coverslip on the bottom of each well in aliquots of 100 μL/well. Cells were allowed to adhere to the coverslips (at 37 °C) for 20 minutes and non-adherent cells were washed with warm PBS. Adherent cells were incubated for 1 hour and forty minutes in complete medium, followed by addition of recombinant 500 nM of either RmS-3, RmS-6, RmS-17 or no serpin or then 106 zymosan particles opsonized with mouse serum. Cells were allowed to phagocyte for 40 minutes and then washed twice with PBS. Coverslips were immediately removed and stained with hematoxylin-eosin (Newprov Kit, Pinhais, PR, Brazil). Phagocytosis was evaluated by light microscopy (Zeiss Axiolab – Zeiss, Oberkochen, Germany). Percentage of phagocytosis was determined by the number of macrophages that had three or more zymosan particles in each 100 cells (Peres et al., 2005).
2.8. Spleen cell cultures
Spleens from naïve mice were aseptically removed and transferred into tubes containing 5 mL of RPMI 1640 medium. The organ was pressed through a 40-μm-pore-size cell strainer (BD Falcon, Franklin Lakes, NJ, USA) with the aid of a sterile syringe plunger. Cells were centrifuged at 300 × g for 5 min at 4 °C and, after discarding the supernatant, the red blood cells were lysed by ACK lysis buffer (Gibco Invitrogen). After further washes, the cells were diluted in Turk’s solution, counted in a Neubauer chamber, suspended at 5 × 106 cells/mL in complete medium and distributed into sterile 96-well plates in aliquots of 100 μL/well.
2.9. Resazurin reduction assay
Resazurin reduction assay was employed to evaluate metabolically active spleen cells (Sá-Nunes et al., 2009; Sá-Nunes et al., 2007b). Spleen cells were prepared as described earlier and maintained in complete medium (control group) or preincubated with R. microplus serpins (10–1000nM) for 1 h followed by stimulation with concanavalin A (Con A - Sigma-Aldrich) at 1 μg/mL final concentration for 72 h. The metabolic activity of the cells was evaluated by adding resazurin (10 μg/mL final concentration) to all wells in the last 24 h incubation, followed by reading the culture absorbance at OD570nm and OD600nm in a plate reader (SpectraMax M3, Molecular Devices, San José, CA, USA) and the results are expressed as the difference between the readings as previously described (Bizzarro et al., 2013).
2.10. Cytokine determination
The cell-free supernatants of macrophages and lymphocyte cultures described above were collected and the levels of TNF-α, IL-12p40 and IL-6 (for macrophages), and IFN-γ (for lymphocytes) were determined by BD OptEIA ELISA Sets according to manufacturer’s instructions (BD Biosciences). The detection limit for each cytokine analyzed was 31.2 pg/mL.
2.11. Lymphocyte proliferation
Spleen cells were prepared as described earlier and stained with CFSE as previously described (Quah et al., 2007). Briefly, a suspension containing 107 cells/mL were labeled with 1 μM of CFSE (CellTrace CFSE Cell Proliferation kit – Invitrogen, Eugene, OR, USA) diluted in 1 mL of PBS. Cells were incubated for 5 min at room temperature, washed 3 times with PBS, suspended at 2 × 106 cells/mL. Cells were distributed in 24-well plates (500 μL/well), followed by preincubation with R. microplus serpins (1000 nM) for 1 h and stimulation with suboptimal (0.5 μg/mL) and optimal (1 μg/mL) concentrations of Con A for 72 h. Cells were then stained at 4 °C for 30 min with fluorochromeconjugated anti-mouse CD3, CD4 and CD8 monoclonal antibodies, acquired by a FACSCanto II flow cytometer and analyzed by the FlowJo software as described earlier.
2.12. Assessment of cell viability
Spleen cells were prepared as described earlier and maintained in complete medium or incubated with the R. microplus serpins (1000 nM) for 2 h in polypropylene round-bottom tubes (17 × 100 mm) at 37 °C and 5% CO2. As an internal control, a group incubated with the salivary gland extract (SGE) of Aedes aegypti, a preparation known to decrease both metabolic activity and proliferation of lymphocytes through cell death (Bizzarro et al., 2013), was included in the assay. Then, cells were washed with flow cytometry buffer (PBS containing 1% FBS), transferred to flow cytometry tubes (12 × 75 mm) and stained with fluorescence-conjugated anti-CD3, anti-CD4 and anti-CD8 monoclonal antibodies (BioLegend) diluted in flow cytometry buffer for 30 min at 4 °C in the dark. Cells were then washed twice with annexin buffer (10 mM HEPES, 140 mM NaCl, 0.25 mM CaCl) and centrifuged at 300 × g for 5 min at 4 °C. The cell pellet was resuspended in 100 μL of annexin buffer and 5 μL of annexin V-FITC (BioLegend) were added to each sample, which was then incubated in the dark for 10 min at room temperature. Sample acquisition was performed by a FACSCanto II flow cytometer (BD Biosciences) to evaluate the percentage of annexin V+ cells. Data was analyzed using the FlowJo software, version 10.0.5 (Tree Star Inc., Ashland, OR, USA).
2.13. Statistical analysis
For the comparison of the experimental groups, Student’s t test or analysis of variance (ANOVA) followed by Tukey as a post-test were used. A p value ≤ 0.05 was considered statistically significant.
3. Results
3.1. rRmS-3, rRmS-6 and rRmS-17 reduce vascular permeability induced by inflammatory stimuli
Enzymatic assays showed that RmS-3 inhibits rMCP-1 by 87% in vitro (Fig. 1). The identity of enzyme was confirmed by mass spectrometry analyses of a rat peritoneum extract (Supplementary Fig. S1). Together with the previous findings showing that recombinant RmS-3, RmS-6 and RmS-17 have as biochemical targets chymase and trypsin-like proteases (Tirloni et al., 2016), our results suggest a potential role of these serpins on inflammatory conditions. Thus, in order to further evaluate the in vivo activities of the serpins, two different models of acute inflammation were employed in rats, according to the inhibitory profile of each serpin. Strikingly, RmS-3 was shown to significantly reduce the vascular permeability induced in the skin by compound 48/80 (p = 0.0007 - Fig. 2A–B), a polymer that promotes mast cell degranulation. Similarly, both RmS-6 (Fig. 2C–D) and RmS-17 (Fig. 2E–F) decreased vascular permeability in the skin induced by formalin (p = 0.0009 and p = 0.0002, respectively), a preparation known to activate trypsin-like proteases.
Fig. 1.
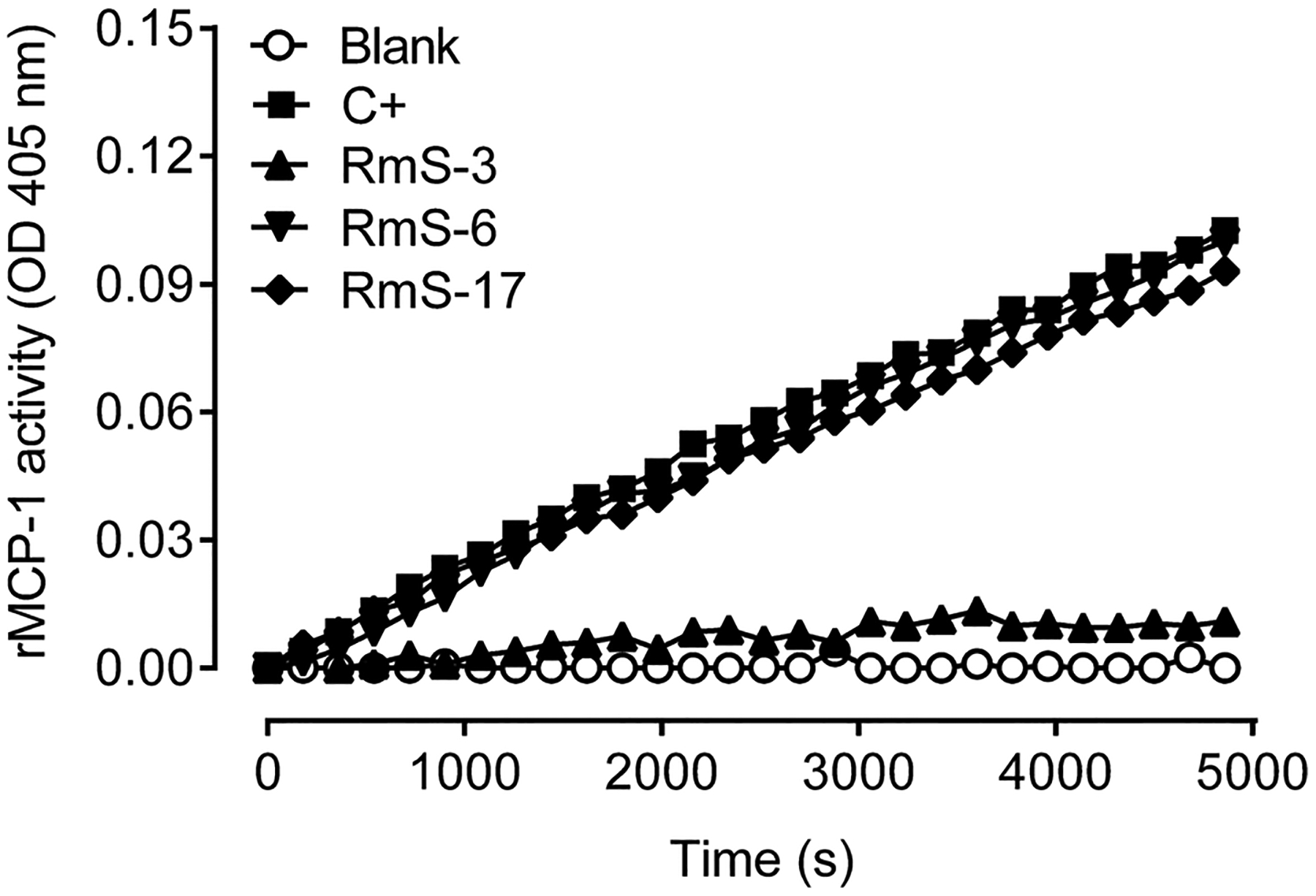
Rhipicephalus microplus serpin RmS-3, but not RmS-6 or RmS-17, inhibits rat MCP-1 (rMCP-1) protease activity. The activity was measured in the presence of each serpin (1000 nM) using a specific colorimetric substrate as described in the Material and Methods.
Fig. 2.
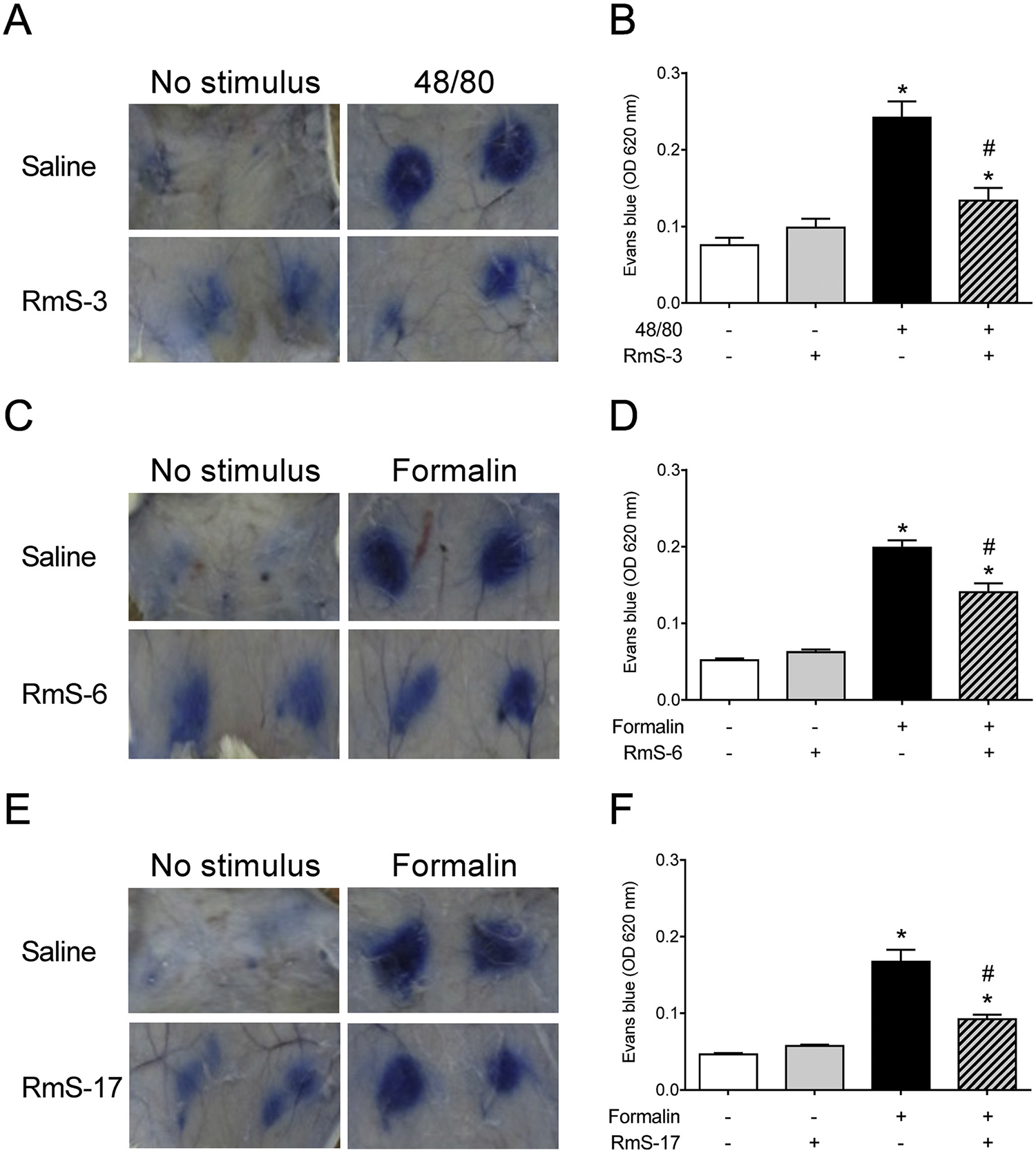
Rhipicephalus microplus serpins decrease vascular permeability induced by inflammatory stimuli. Wistar rats were injected i.v. with Evans blue dye followed by i.d. inoculation of saline, recombinant RmS-3, RmS-6 or RmS-17 and received inflammatory stimuli (compound 48/80 or 2% formalin) in the presence or absence of serpins. After 1 h, animals were euthanized and an area of skin that included the entire injection sites was carefully removed and photographed (A, C, E). Evans blue dye spots on skin were excised and the dye was extracted and measured at OD620nm (B, D, F). *p < 0.05 versus control group (skin inoculated with saline); #p < 0.05 versus “48/or “formalin” group.
3.2. rRmS-3, rRmS-6 and rRmS-17 do not affect macrophage biology
The ability of macrophages to phagocyte opsonized zymosan particles was not altered in the presence of these 3 serpins (Supplementary Fig. S2A). When compared with cells maintained in medium only, the activation with IFN-γ plus LPS induced a significant production of NO, as expected. The incubation of macrophages with increasing concentrations of RmS-3, RmS-6 and RmS-17 prior to IFN-γ plus LPS activation did not affect NO production, even at the higher concentration used (Supplementary Fig. S2B). Regarding inflammatory cytokines TNF-α, IL-12p40 and IL-6, none of the serpins was able to interfere with their production by activated macrophages (Supplementary Fig. S2C–E, respectively). Of note, cells cultured in the presence of the serpins produced almost undetectable levels of NO and cytokines (data not shown).
3.3. rRmS-3, rRmS-6 and rRmS-17 selectively affect metabolic activity, IFN-γ production and proliferation of lymphocytes without inducing cell death
Next, the role of RmS-3, RmS-6 and RmS-17 on parameters associated with T lymphocyte biology was assessed. None of the serpins changed the basal metabolic activity of the spleen cells maintained in medium only. When stimulated with Con A, however, spleen cells presented a partial decrease in their metabolic activity in the presence of the highest concentration of RmS-3 (1000 nM - p < 0.05), while RmS-6 had no effect on it (Fig. 3A and 3B, respectively). Under the same experimental conditions, the metabolic activity of Con A-stimulated spleen cells was decreased in a concentration-response manner in the presence of RmS-17, with a significant effect at 300 nM and 1000 nM (p < 0.05 - Fig. 3C). Regarding Con A-induced IFN-γ production, a similar profile was observed: RmS3 partially inhibited the cytokine production at 1000 nM (Fig. 3D), RmS6 did not change its production (Fig. 3E), and RmS17 significantly inhibited the cytokine production (p < 0.05) at both 300 nM and 1000 nM (Fig. 3F).
Fig. 3.
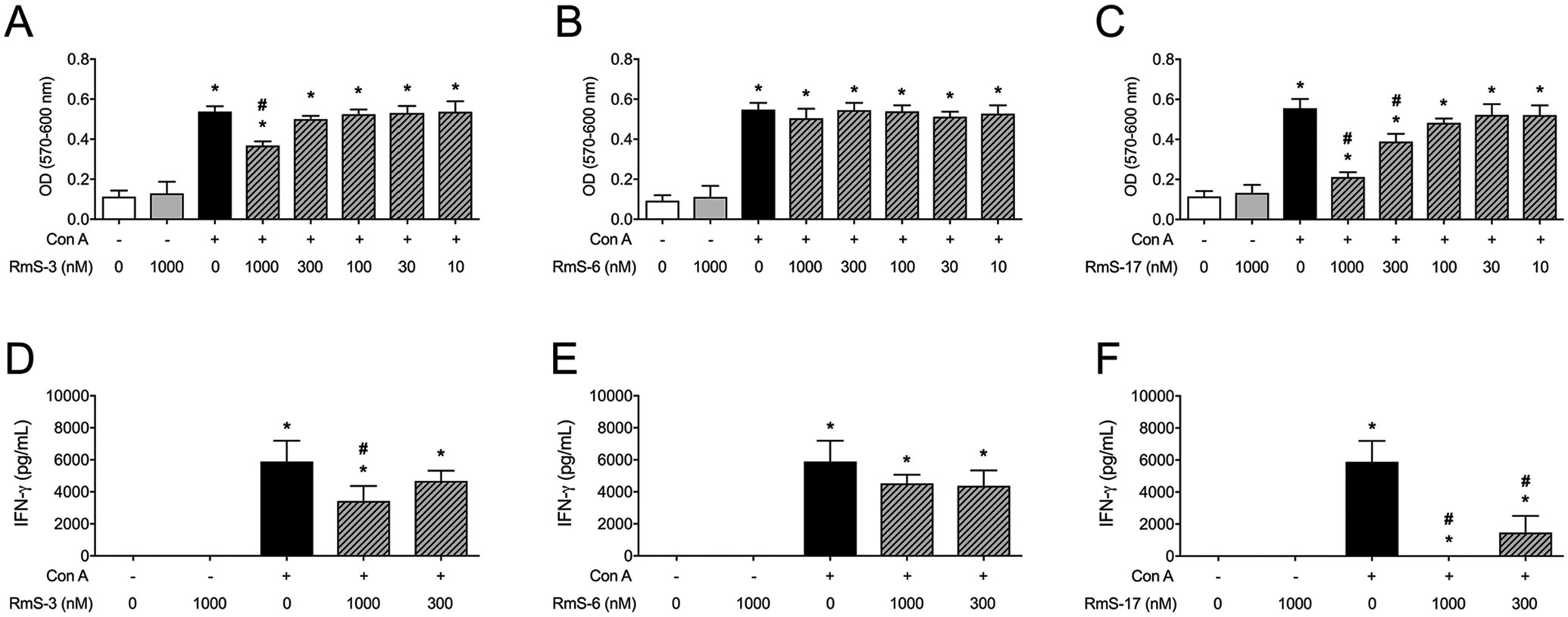
Rhipicephalus microplus serpins differentially affect the metabolic activity and the IFN-γ production of murine T cells. Spleen cells were incubated with medium only or with different concentrations of each serpin (10 – 1000 nM) for 1 h and stimulated or not with Con A (1 μg/mL). The metabolic activity was evaluated by absorbance at 570 and 600 nm (A-C). The IFN-γ production was evaluated by ELISA (D-F). Results are expressed as the mean ± SEM. *p < 0.05 versus control group (cells incubated with medium only); #p < 0.05 versus “Con A” group.
In order to evaluate if the changes in the lymphocyte metabolic activity were associated with impaired cell proliferation, a CFSE dilution assay was evaluated by flow cytometry. As expected, naïve T lymphocytes do not proliferate when incubated with medium (Fig. 4A) or in the presence of the serpins only (Fig. 4B, 4C and 4D for RmS-3, RmS-6 and RmS-17, respectively). Under suboptimal activation conditions, T lymphocytes presented a weak proliferation (Fig. 4E) that was partially inhibited in the presence of RmS-3 (Fig. 4F), did not change in the presence of RmS-6 (Fig. 4G) and was completely inhibited in the presence of RmS-17 (Fig. 4H). However, under optimal activation conditions, almost all lymphocytes proliferated (Fig. 4 I - p < 0.05) and this robust proliferation was barely affected in the presence of RmS-3 or RmS-6 (Fig. 4J and 4 K, respectively). Nevertheless, the presence of RmS-17 inhibited Con A-induced lymphocyte proliferation (Fig. 4L).
Fig. 4.
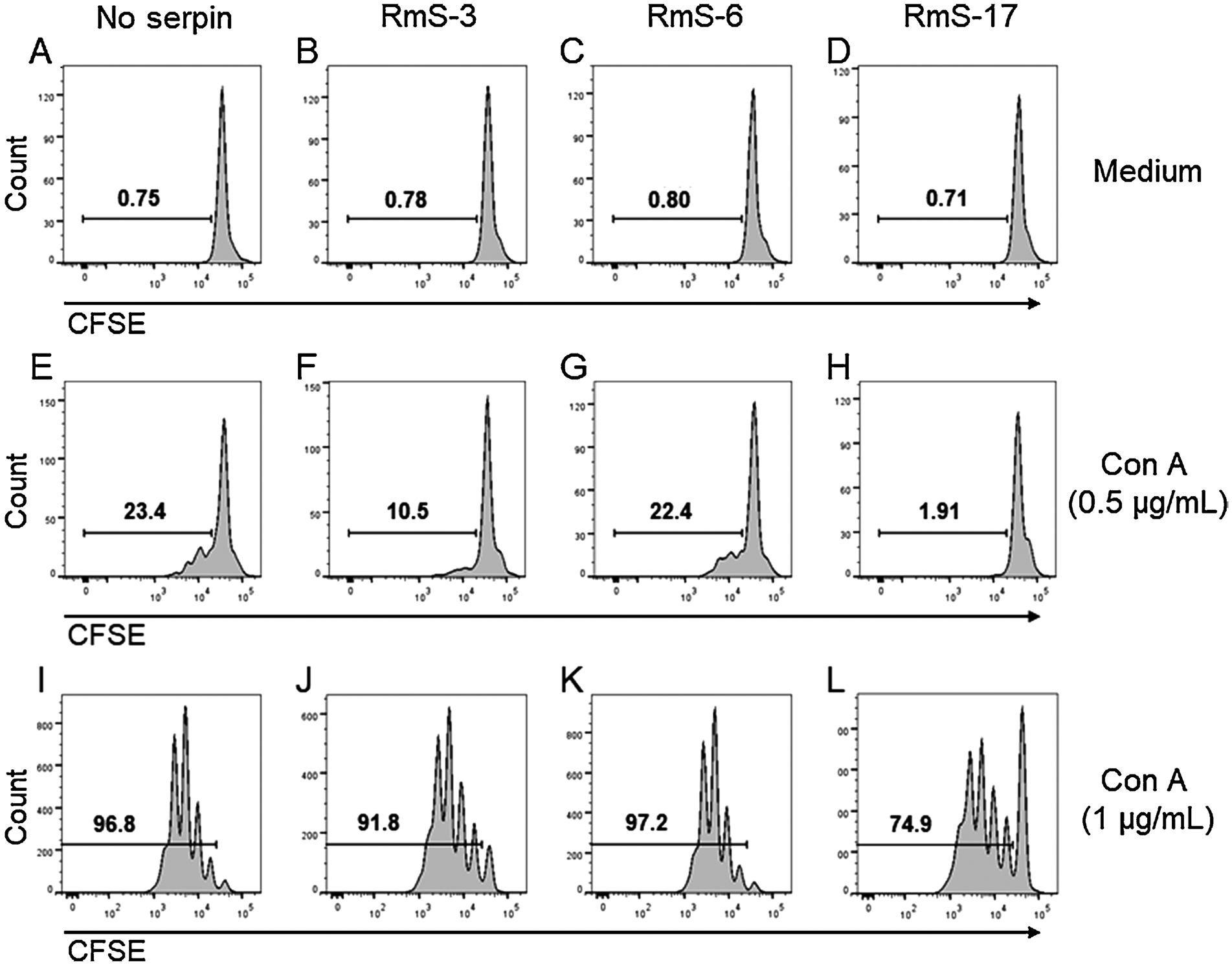
Rhipicephalus microplus serpins differentially affect proliferation of murine T cells. Spleen cells were collected and CFSE-stained, incubated with medium only or with the serpins (1 μM each) for 1 h and stimulated or not with suboptimal (0.5 μg/mL) or optimal (1 μg/mL) concentrations of Con A. Proliferation was determined by flow cytometric analysis of CFSE dilution as described in Material and Methods.
Subsequently, it was evaluated whether the decreased metabolic activity/proliferation of lymphocytes in the presence of R. microplus serpins was due to cell toxicity, by determining the cell death in the presence of the proteins. Annexin V staining in CD4+ T cells showed that compared with cells maintained in medium only (Fig. 5A), the incubation of T lymphocytes with RmS-3 (Fig. 5B), RmS-6 (Fig. 5C) or RmS-17 (Fig. 5D) did not induce exposure of phosphatidylserine on the outer membrane of the cells. On the other hand, the presence of A. aegypti SGE, a negative internal control, induced around 50% cell death under the same conditions (Fig. 5E). Similar results were achieved in CD8+ T cells: compared to medium incubation (Fig. 5F), none of the serpins changed annexin V staining (Fig. 5G–I) while A. aegypti SGE induced increased cell death (Fig. 5J).
Fig. 5.
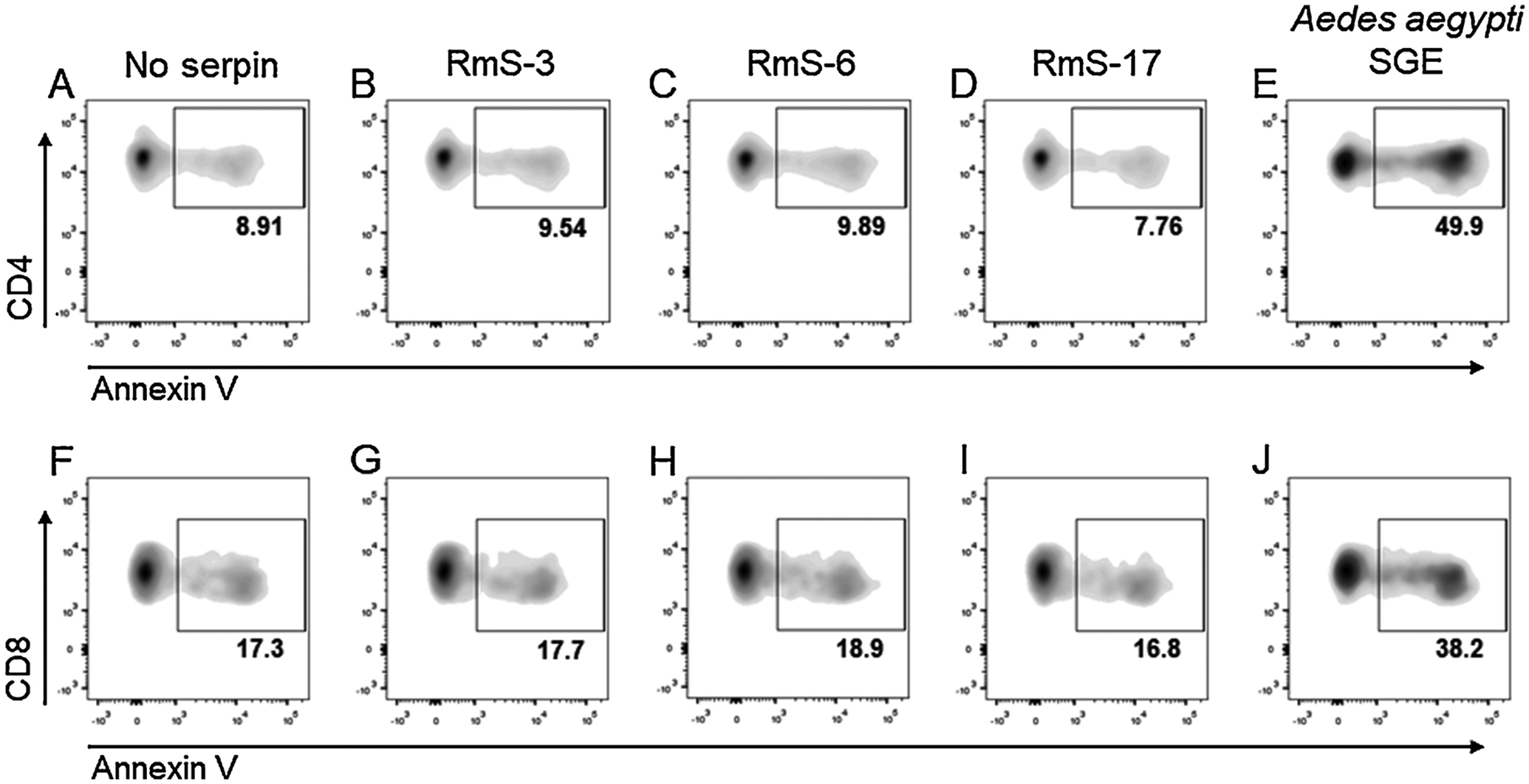
Rhipicephalus microplus serpins do not affect lymphocyte viability. Spleen cells were incubated with medium only, with each serpin (1000 nM) or with Aedes aegypti salivary gland extract (SGE – 10 μg/mL) for 2 h. Cells were stained with fluorescence-conjugated anti-CD3, anti-CD4 and anti-CD8 monoclonal antibodies followed by annexin V and acquired by a flow cytometer to evaluate the percentage of annexin V+ cells.
4. Discussion
The serpin superfamily comprises thousands of proteins with similar structure but different functions, found in genomes of all kingdoms (Gettins, 2002; Silverman et al., 2010). In arthropods, the first serpin biochemically characterized was from the hemolymph of the silkworm Bombyx mori (Sasaki and Kobayashi, 1984). Since then, a growing number of arthropod serpins have been identified in different tissues and fluids, presenting a wide range of functions in development, wound healing, immunity, melanization, antimicrobial and intracellular signaling, among others (Meekins et al., 2017). In ticks, salivary serpins are of special interest because of their potential ability to modulate vertebrate host’s hemostasis and immunity during the blood meal, and a number of recent reviews have covered important aspects of these host-parasite interactions (Chmelař et al., 2017; Meekins et al., 2017; Parizi et al., 2018). However, while a substantial number of studies focused on the description of the biochemical targets of tick salivary serpins, less is known about their role on the host’s inflammation and immunity. The present study attempted to address this point by evaluating the activities of RmS-3, Rm-S6 and RmS-17 on several parameters of vertebrate innate and adaptive immune system.
Previous evidence has shown that RmS-3, RmS-6 and RmS-17 inhibit trypsin-like proteases and chymase (Tirloni et al., 2016), which are abundantly present in mast cell granules and released upon cell activation (Pejler et al., 2010). Mast cells reside in the skin and are present at early stages of an acute inflammatory response, such that caused by the introduction of of the tick mouthparts following attachment to the host. This suggests that tick salivary serpins might attenuate mast cell-induced inflammation and could contribute to the activities of tick saliva in modulating these cells, since they are involved in the host responses against ticks (Engracia Filho et al., 2006; Matsuda et al., 1987, 1985).
Mast cell chymase affects inflammation at different levels, activating the cleavage of pro-inflammatory cytokines/chemokines and of protease-activated receptor 2 (PAR-2), degradation of endothelial cell-cell contacts, activation of extracellular matrix–degrading enzymes, and recruitment of eosinophils/neutrophils (Pejler et al., 2010). Our results show that RmS-3 inhibits rat peritoneum-derived rMCP-1, the main chymase produced by connective tissue–type rat mast cells in the peritoneum. RmS-3 also reduces vascular permeability induced by compound 48/80 which is able to induce plantar mast cell degranulation accompanied by thermal hyperalgesia, tissue edema, and neutrophil influx (Chatterjea et al., 2012). Taking altogether, these results suggest that RmS-3 can significantly modulate early steps of inflammatory responses that occur after tick infestations by inhibiting chymase, released by mast cell activation.
RmS-6 and RmS-17 reduced formalin-induced vascular extravasation. The injection of formalin into the mouse paw locally releases several forms of active trypsin-like serine proteases. Trypsin-like proteases are expressed in the nervous system and in epithelial tissues where they are the most powerful activators of PAR-2, indicating that they are important factors in neurogenic inflammation and pain in the skin (Cattaruzza et al., 2014; Knecht et al., 2007). These proteases generate PAR-derived peptides and activate cells via PAR-2-dependent mechanism, resulting in an acute inflammatory response characterized by edema formation in the paw (Cattaruzza et al., 2014; Knecht et al., 2007). Considering this, tick injection of RmS-6 and RmS-17 into the feeding site could interfere with serine protease-derived pro-inflammatory and algesic responses in the skin during tick feeding.
The immunomodulatory role of serpins was described in many organisms including mammals, birds, worms, plants and insects, presenting different mechanism of action and targeted cells (Silverman et al., 2010). Regarding arthropods, despite a reasonably common in vitro inhibition of macrophage activation (Brake and Pérez De León, 2012; Chen et al., 2012; Kopecký and Kuthejlová, 1998; Kuthejlová et al., 2001; Rodrigues et al., 2018; Urioste et al., 1994) and lymphocyte proliferation (Anguita et al., 2002; Bergman et al., 1995; Ferreira and Silva, 1998; Gillespie et al., 2001; Kotsyfakis et al., 2006; Schoeler et al., 2000; Turni et al., 2004; Urioste et al., 1994; Wang et al., 2017) caused by tick saliva, SGE or some of its constituents, studies on the role of tick salivary serpins in the biology of these cells are scarce. We demonstrated that RmS-3 and RmS-17 from R. microplus, has an impact in T lymphocytes by decreasing metabolism, proliferation and cytokine production. Similar activities were found in salivary serpins described in other arthropods. Iris was the first serpin shown to directly inhibit the proliferation of murine spleen cell stimulated by Con A and the production of IFN-γ by human PBMC stimulated by PHA, CD3/CD28 or LPS (Leboulle et al., 2002). Iris was later described to bind monocytes/macrophages and decrease the production of TNF-α induced by Toll-like receptor agonists in vitro, in addition to protect mice against LPS-induced toxic shock in vivo (Prevot et al., 2009). IRS-2, the second I. ricinus salivary serpin described, indirectly inhibited lymphocyte differentiation to a Th17 profile, but this phenotype was due to an interference with IL-6/STAT-3 signaling pathway on dendritic cells (Páleníková et al., 2015). More recently, two salivary serpins from H. longicornis (HlSerpin-a and HlSerpin-b) were characterized and shown to inhibit the production of inflammatory cytokines triggered by LPS in bone marrow-derived macrophages (Wang et al., 2019). Our work shows that RmS-17, and RmS-3 to a lesser degree, are now members of a selective group of T cell inhibitory serpins found in tick saliva displaying no effect on macrophage activation. The exact mechanism of action of such selective activity remains to be elucidated, but it differs from that described for A. aegypti salivary components since no cell death is involved in the process (Bizzarro et al., 2013).
The present work unveils the multifunctional role of R. microplus salivary serpins on vertebrate immunity. Based on their biochemical targets, we revealed the anti-inflammatory activities of RmS-3, RmS-6 and RmS-17 as inhibitors of mast cell proteases. In addition, RmS-17 presented a potent inhibition of metabolic activity, IFN-γ production and proliferation of lymphocytes without promoting cell death, a phenotype that is partially shared with RmS-3. Knowing that inflammation and adaptive immune responses to protect the host against pathogens transmitted by R. microplus, the salivary serpins may provide a strong immunomodulatory microenvironment at the skin that in turn facilitates pathogen transmission. The biological functions of these serpins on role of immune cells in many inflammatory and autoimmune disorders suggest the potential use of the R microplus serpins as a potential source of immunomodulators to treat these conditions.
Supplementary Material
Acknowledgments
The authors would like to thank Sandra Alexandre (Departamento de Imunologia, Instituto de Ciências Biomédicas, Universidade de São Paulo) for technical assistance and Pedro Ferrari Dalberto and Cristiano Bizarro from Pontifícia Universidade Católica do Rio Grande do Sul for the mass spectrometry analysis.
Funding
This study was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES): Finance Code 001, Grant # 88881.068421/2014-01 (PROCAD) and 23038.005296/2014-37 (PGCI); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq): Grant # 134636/2015-5, 573959/2008-0 (INCT-EM), 465678/2014-9 (INCT-EM), 400603/2012-8 (PVE), 405763/2018-2, 441092/2016-0, and 302360/2018-2; Núcleo de Pesquisa em Moléculas Bioativas de Artrópodes Vetores (NAP-MOBIARVE): Grant # 12.1.17661.1.7 (Brazil).
Abbreviations:
- FBS
fetal bovine serum
- Iris
Ixodes ricinus immunosuppressor
- IRS-2
Ixodes ricinus serpin-2
- PAR-2
protease-activated receptor 2
- PBS
phosphate-buffered saline
- rMCP-1
rat mast cell protease-1
- RmS
Rhipicephalus microplus serpin
- SGE
salivary gland extract
Footnotes
Competing interests
The authors declare that they have no competing interests.
CRediT authorship contribution statement
Mariana L. Coutinho: Conceptualization, Methodology, Formal analysis, Visualization, Writing - original draft, Writing - review & editing. Bruna Bizzarro: Conceptualization, Methodology, Formal analysis, Visualization, Writing - original draft, Writing - review & editing. Lucas Tirloni: Conceptualization, Methodology, Formal analysis, Visualization, Writing - original draft, Writing - review & editing. Markus Berger: Methodology, Formal analysis, Visualization, Writing -original draft, Writing - review & editing. Carlo Jose Freire Oliveira: Conceptualization, Methodology, Formal analysis, Visualization, Writing - original draft, Writing - review & editing. Anderson Sá-Nunes: Supervision, Funding acquisition, Writing - original draft, Writing - review & editing, Visualization, Project administration. Itabajara Silva Vaz: Supervision, Funding acquisition, Writing - original draft, Writing - review & editing, Visualization, Project administration.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ttbdis.2020.101425.
References
- Anguita J, Ramamoorthi N, Hovius JWR, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincón M, Kantor FS, Fikrig E, 2002. Salp15, an Ixodes scapularis salivary protein, inhibits CD4+ T cell activation. Immunity 16, 849–859. 10.1016/S1074-7613(02)00325-4. [DOI] [PubMed] [Google Scholar]
- Bergman DK, Ramachandra RN, Wikel SK, 1995. Dermacentor andersoni: salivary gland proteins suppressing T-lymphocyte responses to concanavalin A in vitro. Exp. Parasitol 81, 262–271. 10.1006/expr.1995.1117. [DOI] [PubMed] [Google Scholar]
- Bizzarro B, Barros MS, MacIel C, Gueroni DI, Lino CN, Campopiano J, Kotsyfakis M, Amarante-Mendes GP, Calvo E, Capurro ML, Sá-Nunes A, 2013. Effects of Aedes aegypti salivary components on dendritic cell and lymphocyte biology. Parasit. Vectors 6. 10.1186/1756-3305-6-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake DK, Pérez De León AA, 2012. Immunoregulation of bovine macrophages by factors in the salivary glands of Rhipicephalus microplus. Parasit. Vectors 5. 10.1186/1756-3305-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breijo M, Esteves E, Bizzarro B, Lara PG, Assis JB, Rocha S, Pastro L, Fernández C, Meikle A, Sá-Nunes A, 2018. Hematobin is a novel immunomodulatory protein from the saliva of the horn fly Haematobia irritans that inhibits the inflammatory response in murine macrophages. Parasit. Vectors 11. 10.1186/s13071-018-3017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaruzza F, Amadesi S, Carlsson JF, Murphy JE, Lyo V, Kirkwood K, Cottrell GS, Bogyo M, Knecht W, Bunnett NW, 2014. Serine proteases and protease-activated receptor 2 mediate the proinflammatory and algesic actions of diverse stimulants. Br. J. Pharmacol 171, 3814–3826. 10.1111/bph.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjea D, Wetzel A, Mack M, Engblom C, Allen J, Mora-Solano C, Paredes L, Balsells E, Martinov T, 2012. Mast cell degranulation mediates compound 48/80-induced hyperalgesia in mice. Biochem. Biophys. Res. Commun 425, 237–243. 10.1016/j.bbrc.2012.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Severo MS, Sohail M, Sakhon OS, Wikel SK, Kotsyfakis M, Pedra JH, 2012. Ixodes scapularis saliva mitigates inflammatory cytokine secretion during Anaplasma phagocytophilum stimulation of immune cells. Parasit. Vectors 5. 10.1186/1756-3305-5-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmelar J, Oliveira CJ, Rezacova P, Francischetti IMB, Kovarova Z, Pejler G, Kopacek P, Ribeiro JMC, Mares M, Kopecky J, Kotsyfakis M, 2011. A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation. Blood 117, 736–744. 10.1182/blood-2010-06-293241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmelař J, Kotál J, Langhansová H, Kotsyfakis M, 2017. Protease inhibitors in tick saliva: the role of serpins and cystatins in tick-host-pathogen interaction. Front. Cell. Infect. Microbiol 7. 10.3389/fcimb.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engracia Filho JR, Bechara GH, Teodoro RL, 2006. Dermal Mast Cell Counts in F2 Holstein X Gir Crossbred Cattle Artificially Infested With the Tick Boophilus microplus (Acari: Ixodidae). Annals of the New York Academy of Sciences, pp. 476–478. 10.1196/annals.1373.070. [DOI] [PubMed] [Google Scholar]
- Ferreira BR, Silva JS, 1998. Saliva of Rhipicephalus sanguineus tick impairs T cell proliferation and IFN-γ-induced macrophage microbicidal activity. Vet. Immunol. Immunopathol 64, 279–293. 10.1016/S0165-2427(98)00135-4. [DOI] [PubMed] [Google Scholar]
- Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM, 2009. The role of saliva in tick feeding. Front. Biosci 2051. 10.2741/3363. Volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettins PGW, 2002. Serpin structure, mechanism, and function. Chem. Rev 102, 4751–4803. 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- Gillespie RD, Dolan MC, Piesman J, Titus RG, 2001. Identification of an IL-2 binding protein in the saliva of the lyme disease vector tick, ixodes scapularis. J. Immunol 166, 4319–4326. 10.4049/jimmunol.166.7.4319. [DOI] [PubMed] [Google Scholar]
- Grisi L, Leite RC, de, S.Martins JR, Barros A.T.Mde, Andreotti R, Cançado PHD, León A.A.Pde, Pereira JB, Villela HS, 2014. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev. Bras. Parasitol. Veterinária 23, 150–156. 10.1590/S1984-29612014042. [DOI] [PubMed] [Google Scholar]
- Heinze DM, Carmical JR, Aronson JF, Thangamani S, 2012a. Early immunologic events at the tick-host interface. PLoS One 7, e47301. 10.1371/journal.pone.0047301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze DM, Wikel SK, Thangamani S, Alarcon-Chaidez FJ, 2012b. Transcriptional profiling of the murine cutaneous response during initial and subsequent infestations with Ixodes scapularis nymphs. Parasit. Vectors 5. 10.1186/1756-3305-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongejan F, Uilenberg G, 2004. The global importance of ticks. Parasitology 129, S3–S14. 10.1017/S0031182004005967. [DOI] [PubMed] [Google Scholar]
- Kim TK, Tirloni L, Radulovic Z, Lewis L, Bakshi M, Hill C, da Silva Vaz I, Logullo C, Termignoni C, Mulenga A, 2015. Conserved Amblyomma americanum tick Serpin19, an inhibitor of blood clotting factors Xa and XIa, trypsin and plasmin, has anti-haemostatic functions. Int. J. Parasitol 45, 613–627. 10.1016/j.ijpara.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht W, Cottrell GS, Amadesi S, Mohlin J, Skåregärde A, Gedda K, Peterson A, Chapman K, Hollenberg MD, Vergnolle N, Bunnett NW, 2007. Trypsin IV or mesotrypsin and p23 cleave protease-activated receptors 1 and 2 to induce inflammation and hyperalgesia. J. Biol. Chem 282, 26089–26100. 10.1074/jbc.M703840200. [DOI] [PubMed] [Google Scholar]
- Kopecký J, Kuthejlová M, 1998. Suppressive effect of Ixodes ricinus salivary gland extract on mechanisms of natural immunity in vitro. Parasite Immunol. 20, 169–174. [PubMed] [Google Scholar]
- Kotsyfakis M, Sá-Nunes A, Francischetti IMB, Mather TN, Andersen JF, Ribeiro JMC, 2006. Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick ixodes scapularis. J. Biol. Chem 281, 26298–26307. 10.1074/jbc.M513010200. [DOI] [PubMed] [Google Scholar]
- Kuthejlová M, Kopecký J, Štěpáová G, Macela A, 2001. Tick salivary gland extract inhibits killing of Borrelia afzelii spirochetes by mouse macrophages. Infect. Immun 69, 575–578. 10.1128/IAI.69.1.575-578.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboulle G, Crippa M, Decrem Y, Mejri N, Brossard M, Bollen A, Godfroid E, 2002. Characterization of a novel salivary immunosuppressive protein from Ixodes ricinus ticks. J. Biol. Chem 277, 10083–10089. 10.1074/jbc.M111391200. [DOI] [PubMed] [Google Scholar]
- Maruyama SR, Garcia GR, Teixeira FR, Brandão LG, Anderson JM, Ribeiro JMC, Valenzuela JG, Horackova J, Veríssimo CJ, Katiki LM, Banin TM, Zangirolamo AF, Gardinassi LG, Ferreira BR, De Miranda-Santos IKF, 2017. Mining a differential sialotranscriptome of Rhipicephalus microplus guides antigen discovery to formulate a vaccine that reduces tick infestations. Parasit. Vectors 10. 10.1186/s13071-017-2136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Fukui K, Kiso Y, Kitamura Y, 1985. Inability of genetically mast cell-deficient W/W v mice to acquire resistance against larval haemaphysalis longicornis ticks. J. Parasitol 71, 443. 10.2307/3281535. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Nakano T, Kiso Y, Kitamura Y, 1987. Normalization of anti-tick response of mast cell-deficient W/W v mice by intracutaneous injection of cultured mast cells. J. Parasitol 73, 155. 10.2307/3282361. [DOI] [PubMed] [Google Scholar]
- Medeiros AI, Sá-Nunes A, Soares EG, Peres CM, Silva CL, Faccioli LH, 2004. Blockade of endogenous leukotrienes exacerbates pulmonary histoplasmosis. Infect. Immun 72, 1637–1644. 10.1128/IAI.72.3.1637-1644.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meekins DA, Kanost MR, Michel K, 2017. Serpins in arthropod biology. Semin. Cell Dev. Biol 62, 105–119. 10.1016/j.semcdb.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renné T, 2009. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell 139, 1143–1156. 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Páleníková J, Lieskovská J, Langhansová H, Kotsyfakis M, Chmelař J, Kopeckỳ J, 2015. Ixodes ricinus salivary serpin IRS-2 affects Th17 differentiation via inhibition of the interleukin-6/STAT-3 signaling pathway. Infect. Immun 83, 1949–1956. 10.1128/IAI.03065-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parizi LF, Ali A, Tirloni L, Oldiges DP, Sabadin GA, Coutinho ML, Seixas A, Logullo C, Termignoni C, da Silva Vaz I, 2018. Peptidase inhibitors in tick physiology. Med. Vet. Entomol 10.1111/mve.12276. [DOI] [PubMed] [Google Scholar]
- Pejler G, Rönnberg E, Waern I, Wernersson S, 2010. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood. 10.1182/blood-2010-01-257287. [DOI] [PubMed] [Google Scholar]
- Prevot PP, Beschin A, Lins L, Beaufays J, Grosjean A, Bruys L, Adam B, Brossard M, Brasseur R, Zouaoui Boudjeltia K, Vanhamme L, Godfroid E, 2009. Exosites mediate the anti-inflammatory effects of a multifunctional serpin from the saliva of the tick Ixodes ricinus. FEBS J. 276, 3235–3246. 10.1111/j.1742-4658.2009.07038.x. [DOI] [PubMed] [Google Scholar]
- Quah BJC, Warren HS, Parish CR, 2007. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat. Protoc 2, 2049–2056. 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- Rodrigues V, Fernandez B, Vercoutere A, Chamayou L, Andersen A, Vigy O, Demettre E, Seveno M, Aprelon R, Giraud-Girard K, Stachurski F, Loire E, Vachiéry N, Holzmuller P, 2018. Immunomodulatory effects of Amblyomma variegatum saliva on bovine cells: characterization of cellular responses and identification of molecular determinants. Front. Cell. Infect. Microbiol 7. 10.3389/fcimb.2017.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Valle M, Xu T, Kurscheid S, Lew-Tabor AE, 2015. Rhipicephalus microplusserine protease inhibitor family: annotation, expression and functional characterisation assessment. Parasit. Vectors 8. 10.1186/s13071-014-0605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá-Nunes A, Bafica A, Lucas DA, Conrads TP, Veenstra TD, Andersen JF, Mather TN, Ribeiro JMC, Francischetti IMB, 2007a. Prostaglandin E2 is a major inhibitor of dendritic cell maturation and function in Ixodes scapularis saliva. J. Immunol 179, 1497–1505. 10.4049/jimmunol.179.3.1497. [DOI] [PubMed] [Google Scholar]
- Sá-Nunes A, Medeiros AI, Sorgi CA, Soares EG, Maffei CML, Silva CL, Faccioli LH, 2007b. Gr-1+ cells play an essential role in an experimental model of disseminated histoplasmosis. Microbes Infect. 9, 1393–1401. 10.1016/j.micinf.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Sá-Nunes A, Bafica A, Antonelli LR, Choi EY, Francischetti IMB, Andersen JF, Shi G-P, Chavakis T, Ribeiro JM, Kotsyfakis M, 2009. The immunomodulatory action of sialostatin l on dendritic cells reveals its potential to interfere with auto-immunity. J. Immunol 182, 7422–7429. 10.4049/jimmunol.0900075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Kobayashi K, 1984. Isolation of two novel proteinase inhibitors from hemolymph of silkworm larva, Bombyx mori. Comparison with human serum proteinase inhibitors. J. Biochem 95, 1009–1017. 10.1093/oxfordjournals.jbchem.a134688. [DOI] [PubMed] [Google Scholar]
- Schoeler GB, Bergman DK, Manweiler SA, Wikel SK, 2000. Influence of soluble proteins from the salivary glands of ixodid ticks on the in-vitro proliferative responses of lymphocytes from BALB/c and C3H/HeN mice. Ann. Trop. Med. Parasitol 94, 507–518. 10.1080/00034983.2000.11813570. [DOI] [PubMed] [Google Scholar]
- Silverman GA, Whisstock JC, Bottomley SP, Huntington JA, Kaiserman D, Luke CJ, Pak SC, Reichhart JM, Bird PI, 2010. Serpins flex their muscle: I. Putting the clamps on proteolysis in diverse biological systems. J. Biol. Chem 285, 24299–24305. 10.1074/jbc.R110.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirloni L, Reck J, Terra RMS, Martins JR, Mulenga A, Sherman NE, Fox JW, Yates JR, Termignoni C, Pinto AFM, Vaz IDS, 2014a. Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: a comparison between partially and fully engorged females. PLoS One 9, e94831. 10.1371/journal.pone.0094831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirloni L, Seixas A, Mulenga A, Da Silva Vaz I, Termignoni C, 2014b. A family of serine protease inhibitors (serpins) in the cattle tick Rhipicephalus (Boophilus) microplus. Exp. Parasitol 137, 25–34. 10.1016/j.exppara.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Tirloni L, Kim TK, Coutinho ML, Ali A, Seixas A, Termignoni C, Mulenga A, Da Silva Vaz I, 2016. The putative role of Rhipicephalus microplus salivary serpins in the tick-host relationship. Insect Biochem. Mol. Biol 71, 12–28. 10.1016/j.ibmb.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirloni L, Kim TK, Berger M, Termignoni C, Da Silva Vaz I, Mulenga A, 2019. Amblyomma americanum serpin 27 (AAS27) is a tick salivary anti-inflammatory protein secreted into the host during feeding. PLoS Negl. Trop. Dis 13, e0007660. 10.1371/journal.pntd.0007660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomane K, Konnai S, Niwa A, Githaka N, Isezaki M, Yamada S, Ito T, Takano A, Ando S, Kawabata H, Murata S, Ohashi K, 2016. Identification and the preliminary in vitro characterization of IRIS homologue from salivary glands of Ixodes persulcatus Schulze. Ticks Tick. Dis 7, 119–125. 10.1016/j.ttbdis.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Turni C, Lee RP, Jackson LA, 2004. A comparison of the immunosuppressive effects of salivary gland extracts from two laboratory strains of Boophilus microplus. Int. J. Parasitol 34, 833–838. 10.1016/j.ijpara.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Urioste S, Hall LR, Telford SR, Titus RG, 1994. Saliva of the lyme disease vector, lxodes dammini, blocks cell activation by a nonprostaglandin E 2 -dependent mechanism. J. Exp. Med 180, 1077–1085. 10.1084/jem.180.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Lu X, Guo F, Gong H, Zhang H, Zhou Y, Cao J, Zhou J, 2017. The immunomodulatory protein RH36 is relating to blood-feeding success and oviposition in hard ticks. Vet. Parasitol 240, 49–59. 10.1016/j.vetpar.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Wang F, Song Z, Chen J, Wu Q, Zhou X, Ni X, Dai J, 2019. The immunosuppressive functions of two novel tick serpins, HlSerpin-a and HlSerpin-b, from Haemaphysalis longicornis. Immunology 159, 109–120. 10.1111/imm.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Lew-Tabor A, Rodriguez-Valle M, 2016. Effective inhibition of thrombin by Rhipicephalus microplus serpin-15 (RmS-15) obtained in the yeast Pichia pastoris. Ticks Tick. Dis 7, 180–187. 10.1016/j.ttbdis.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Xu Z, Lin Z, Wei N, Di Q, Cao J, Zhou Y, Gong H, Zhang H, Zhou J, 2019. Immunomodulatory effects of Rhipicephalus haemaphysaloides serpin RHS2 on host immune responses. Parasit. Vectors 12. 10.1186/s13071-019-3607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


