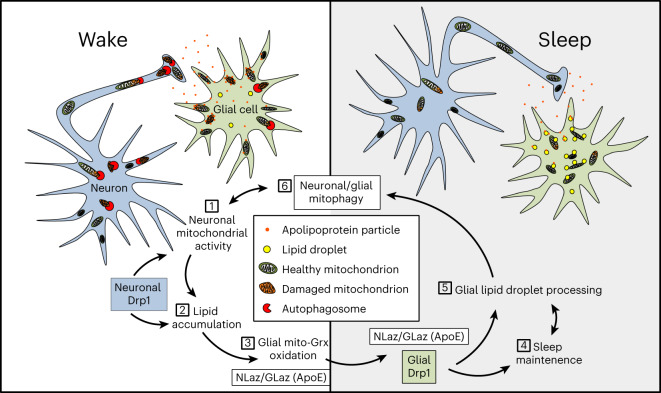
Fig. 7. Model for a sleep-regulated metabolic cycle between neurons and glia.
(1) During wake, mitochondrial energetic activity in neurons results in the production of lipids, which are transferred to glia. (2) As lipids are transferred during wake, glial mitochondria become oxidized, as indicated by mito-roGFP-Grx1 and MitoTimer. Neuronal Drp1 (blue) is required for maximal mitochondrial energetic efficiency and subsequent glial lipid accumulation. (3) Wake-driven glial mitochondrial oxidative stress and lipid accumulation also require the expression of the lipid transfer genes GLaz in glia and NLaz in neurons. Reductions in GLaz or NLaz disrupt lipid droplet dynamics and cause mitochondrial oxidative stress to accumulate in neurons rather than in glia (Fig. 3e). Sustained lipid delivery and subsequent catabolism of lipids in glia, facilitated by neuronal NLaz, glial GLaz, Drp1 and glial mitochondrial β-oxidation (Mcad), are required for daily sleep (4), and, in turn, sleep is necessary for glial lipid catabolism (5). Finally, sleep promotes mitophagy in neurons and glia (6), ensuring the maintenance of a new/healthy population of mitochondria, which are critical for maximally efficient neuronal mitochondrial activity during a new day of wake (1).