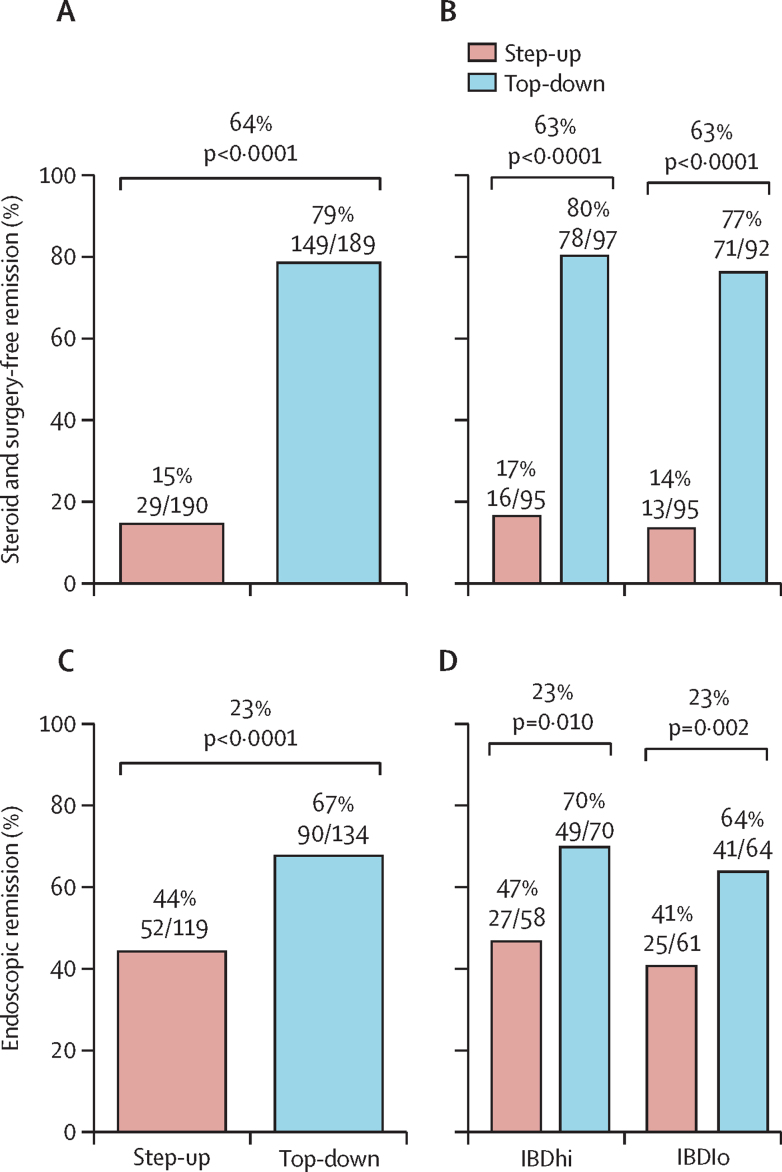
Figure 3.
Primary endpoint and key secondary endpoint
(A) Sustained steroid-free and surgery-free remission until week 48 for treatment groups. (B) Sustained steroid-free and surgery-free remission until week 48 for biomarker–treatment subgroups. (C) Endoscopic remission (absence of ulceration) at week 48 for treatment groups. (D) Endoscopic remission (absence of ulceration) at week 48 for biomarker-treatment subgroups.