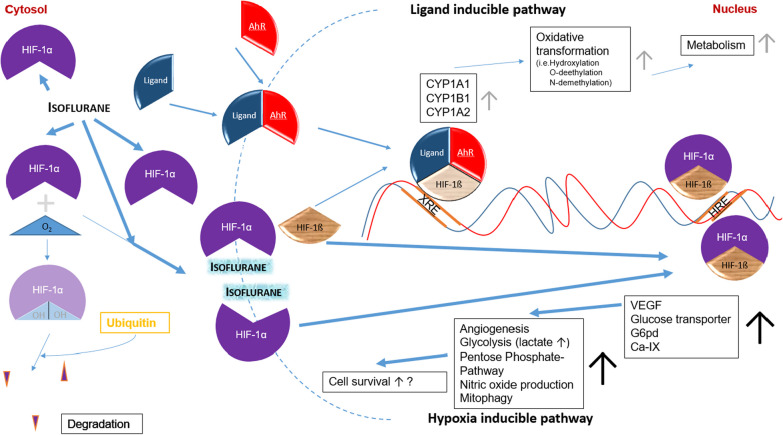
Fig. 2.
Hypothetical network model of isoflurane on oxygen dependent regulation via HIF-1α. Under normoxic conditions, HIF-1α is hydroxylated in the cytosol and degraded in an ubiquitin-dependent manner. Upon hypoxia, less HIF-1α is hydroxylated and degraded. The unhydroxylated HIF-1α translocates to the nucleus and binds to HIF-1ß to form the active transcription factor HIF-1. This binds to hypoxia responsive elements (HRE) and activate the hypoxia-inducible pathway. A reduced availability of HIF-1ß leads to downregulation of the ligand-inducible pathway. This is activated, when a ligand (e.g., polycyclic aromatic hydrocarbons, dioxins or ß-naphthoflavones) binds to the aryl hydrocarbon receptor (AhR), allowing translocation of the complex to the nucleus where it binds to HIF-1ß. The emerged transcription factor binds to xenobiotic responsive elements (XRE) in the promotor region of xenobiotic-degrading enzymes including CYP1A1, CYP1B1 and CYP1A2 enhancing their expression. Therefore, increased binding of HIF-1ß to HIF-1α under hypoxic conditions results in less active ligand/AhR/HIF-1ß transcription factor and a subsequent decrease of CYP1A1, CYP1B1 and CYP1A2 expression. Isoflurane leads to upregulation of HIF-1α protein expression, increased HIF-1α nuclear translocation [35] and DNA binding activity [36]. Isoflurane may cause a reduction of the CYP1A2 enzyme activity, measured by LiMAx, via upregulation of the hypoxia-inducible pathway and subsequent downregulation of the ligand-inducible pathway. VEGF: vascular endothelial growth factor; G6pd: glucose-6-phosphate dehydrogenase; CA-IX: carbonic anhydrase 9