Abstract
Oral microecological dysregulation has been shown to be associated with various immune system disorders. Henoch-schonlein purpura (HSP) is an autoimmune small vessel inflammatory disease in children of uncertain etiology, and studies have suggested that streptococcal infection may be an influential factor in its development. However, the relationship between oral microecological dysregulation and HSP has not been clearly studied so far. In this study, an epidemiological survey on the oral health status of children with HSP was investigated in this paper, and collected dental plaque from four groups of children for 16SrDNA high-throughput sequencing to analyze the composition and changes of oral microbial diversity among different groups. The results showed that the oral health status of children with HSP was poor, except for the incidence of caries in the 5-year-old group, the caries rate and dmfs/DMFS in the 3,4 and 5-year-old groups were higher than the same age in the fourth Chinese Oral Health Epidemiological Survey. Moreover, the development of HSP is accompanied by disturbances in the oral microbiota; a decrease in the number of Firmicutes which producing butyric acid may be closely associated with the development of HSP; changes in the abundance of Streptococcus and Neisseria may be a risk factor for the development of HSP.
Keywords: Henoch-schonlein purpura, Oral microbiota imbalance, Children
1. Introduction
The oral cavity can be defined as the external connection to the respiratory and digestive tracts. Under normal physiological conditions, there is a dynamic balance between oral microecology and the host. While the oral cavity provides a special environment for the growth and reproduction of various microorganisms [1], there are various interactions between microbial communities in the oral cavity, including nutritional competition, synergism, antagonism, and virulence factors [2], which can keep the oral microorganisms healthy under normal physiological conditions. However, the ability of the oral microecology to maintain homeostasis is limited, and disruption of this balance can cause oral or systemic diseases under the action of certain factors such as physical, chemical, host factors, and similar [3]. Oral microflora does not only have an important role in the development of oral diseases such as periodontal disease [4] and oral cancer [5] but is also closely related to systemic diseases such as cardiovascular diseases, rheumatoid arthritis [6], and tumors [7], [8], [9], which have a significant impact on human health.
Henoch-schonlein purpura (HSP) is an IgA-mediated systemic small vessel vasculitis with a predilection for the skin, gastrointestinal tract, joints, and kidneys. It is the most common form of systemic vasculitis in children [10]. A patient was classified as HSP in the presence of purpura or petechiae (mandatory) with lower limb predominance plus one of four criteria: (1) abdominal pain; (2) histopathology (IgA); (3) arthritis or arthralgia; (4) renal involvement. [11]. In recent years, the incidence of HSP has increased, with approximately 90% of affected children being < 10 years of age, with a mean age of 6 years, about 30%-50% of children develop different degrees of renal injury within 4-6 weeks of the onset of the disease. Although most of the children have a favorable prognosis, 1%-17% of children with HSPN develop chronic kidney disease or end-stage renal disease [12]. While the etiology of HSP remains unclear, studies have shown that HSP may be associated with infections, vaccinations, pollen, mosquito bites, food, drugs, and immune factors that cause it [13], [14]. Many reports have shown that infections, especially hemolytic streptococcal infections, may be potential predisposing factors for this disease [15], [16], while 30% of children with HSPN had group A hemolytic streptococcal antigen (nephritis-associated plasmin receptor, NAP1r) deposition in the glomerular tract. However, in other types of nephritis, the NAP1r deposition rate was only 3%, thus indicating that group A hemolytic streptococcal infection is an important predisposing factor for HSP [17].
When the oral micro-ecological balance is disrupted, oral diseases such as dental caries tend to appear. Based on certain immune abnormalities in HSP patients, dental caries as a potential source of infection continuously stimulates the organism to produce corresponding antibodies. Also, the antigen and antibody are combined to form soluble circulating complexes, which are deposited in the renal basement membrane and aggravate renal damage [18]. In their study, Ioue et al. examined 40 children with HSP, finding severe oral diseases, especially caries (70%) and apical periodontitis (5%), where the infected lesions were treated with antimicrobial therapy and root canal therapy. In 31 patients with HSP, dental treatment resulted in complete healing of HSP without nephritis or recurrent episodes, and all patients with HSP achieved clinical remission [19]. This suggested that caries correlates with renal damage in children with HSP [20]; however, no study reported whether the change in oral microflora diversity is related to the development of HSP disease in children.
The aim of this study was to investigate the effect of oral microbiota imbalance on HSP in children by using an oral epidemiological survey and high-throughput sequencing of oral microorganisms.
2. Results
2.1. Survey on the oral health status of children with allergic purpura
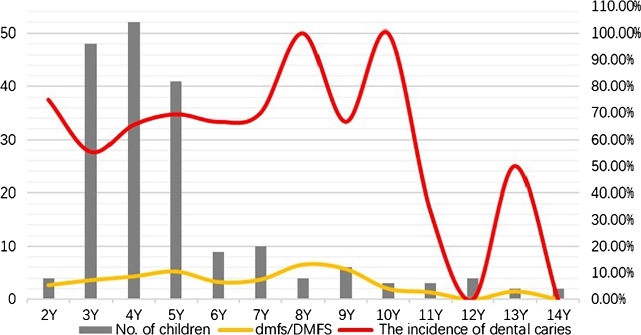
A total of 185 children with HSP diagnosed by the Department of Pediatric, General Hospital of Ningxia Medical University between March 2020 and October 2021 were collected, including 95 males and 90 females. The number of cases, the incidence of dental caries, and dmfs/DMFS in each group are shown in Fig. 1. The age of HSP patients was mainly 3-5 years old, and there were 141 cases of dental caries (48 cases in the 3-year group, 52 cases in the 4-year group, and 41 cases in the 5-year group), accounting for 76.22% of the total number of cases. Detailed analysis showed that the incidence of dental caries in the 3-5 years old HSP children was 65.23% for females and 64.89% for males (), revealing no significant difference in gender.
Figure 1.
The incidence of dental caries, and dmfs/DMFS in HSP children. The gray bars indicate the number of children with HSP at each age, and the yellow lines show the dmfs/DMFS of children with HSP at each age., and the red lines present the incidence of dental caries of children with HSP at each age.
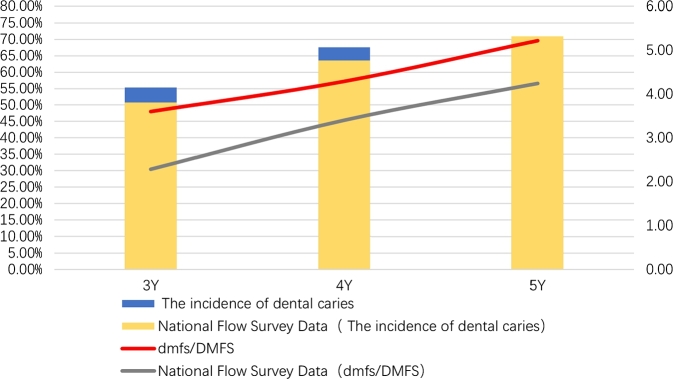
As shown in Fig. 2, the incidence of dental caries in children with HSP was 55.34% in the 3-year-old group, 67.63% in the 4-year-old group, and 69.94% in the 5-year-old group. The caries rates in children with HSP in the 3- and 4-years old groups were higher than the results reported by the fourth Chinese oral health epidemiological survey (50.8% in the 3 years old group and 63.6% in the 4 years old group [21]). However, the incidence of caries in 5-year-old HSP children was lower than that in the fourth National Oral health epidemiological survey (70.90% [21]); no significant difference was observed.
Figure 2.
The incidence of dental caries, and dmfs of HSP children in the 3-5 years old group. The blue bar indicates the caries rate of children with HSP aged 3-5 years, the yellow bar shows the caries rate of children aged 3-5 years in the national epidemiological survey; the red line means the dmfs/DMFS of children with HSP aged 3-5 years, and the gray line represents the dmfs/DMFS of children aged 3-5 years in the national epidemiological survey.
The dmfs/DMFS of HSP children aged 3-5 years were 4.87 in females and 4.94 in males (), revealing no significant difference between males and females. As shown in Fig. 2, dmfs/DMFS of HSP children were 3.60 in the 3-year-old group, 4.29 in the 4-year-old group, and 5.22 in the 5-year-old group, which was higher than the results reported by the fourth Chinese Oral Health Epidemiological Survey (2.28 in the 3-year-old group, 3.40 in the 4-year-old group and 4.24 in the 5-year-old group) [21] (); the observed differences were statistically significant.
2.2. Analysis of oral microbial diversity in children with HSP and healthy children
2.2.1. Basic information on species
We performed high-throughput sequencing of 16SrDNA on all samples using the Illumina MiSeq platform. Finally, we acquired 2937080 high-quality valid sequences with an average length of 423 bp, after which OTU clustering was performed with a coverage index higher than 99.9% and similarity control at 97%, and a total of 1892 OTUs were obtained. The classification statistics yielded 21 phyla, 32 orders, 68 phyla, 126 families, 263 genera, and 1892 species.
2.2.2. Alpha diversity analysis
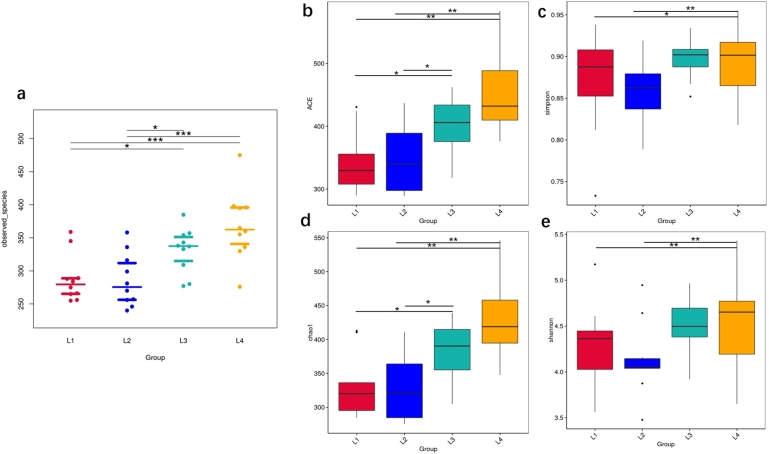
The Tukey test was used for two-way comparisons between groups L1, L2, L3, and L4. A comparative plot of observed species between groups is shown in Fig. 3a. Compared with the L2 group, the Ace (Fig. 3b), Simpson index (Fig. 3c) and Chao1 (Fig. 3d) of the L1 group were smaller, and the Shannon index (Fig. 3e) was larger, indicating that caries may decrease the oral microbial richness based on HSP. Likewise, the trends of these indexes were consistent when comparing the L1 and L3 groups, thus showing that the oral microbial richness of children with caries decreased compared with the healthy population. There was no statistically significant difference between the three groups compared above ().
Figure 3.
Alpha-diversity of oral microorganisms in each sampling methods among four groups. Overall comparison of Alpha-diversity of species among the four groups(a), Boxplots show the ACE diversity index (b), the simpson diversity index (c), the chao1 diversity index (d) and shannon diversity index (e) of each sampling methods among four groups. (Tukey and Wilcox test, ⁎p < 0.05,⁎⁎p < 0.01,⁎⁎⁎p < 0.005).
Moor ever, the Ace, Chao1 index, and Shannon index of the L3 group were smaller than those of the L4 group, and the Simpson index was larger than those of the L4 group, explaining that the oral microbial richness of children with caries decreased compared with that of the healthy population. The same trend results in the L1, L2, and L4 groups suggested that the oral microbial richness of children with HSP was reduced compared with healthy children; the observed difference was statistically significant at (Fig. 3).
2.2.3. Beta diversity analysis
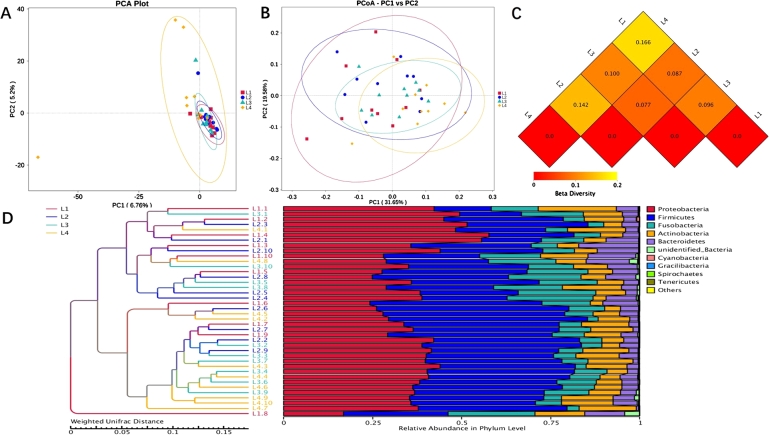
A comparative analysis of the microbial community composition of different groups L1, L2, L3, and L4 was performed. PCA analysis of the sample at the OTU level is shown in Fig. 4a, with the PC1 axis explaining 6.76% and the PC2 axis explaining 5.2% of the overall results. The points that represented the L4 group were more diffuse compared to the other 3 groups, suggesting that children with HSP or caries exhibit decreased oral microbial richness and diversity compared to healthy children. Based on Weighted Unifrac distance, PCoA analysis indicated that the communities of the four groups of samples significantly differed, and the species composition structures were not exactly similar (Fig. 4b). Beta diversity heatmap showed that the coefficient of variation between L1 and L4, L2 and L4 samples were larger, indicating a greater difference in species diversity between children with HSP and healthy children (Fig. 4c). UPGMA clustering analysis was done with the Weighted Unifrac distance matrix (on the left), and the clustering results are presented with the relative abundance of species at the Phylum level for each sample (on the right) in Fig. 4d.
Figure 4.
Beta diversity of oral microorganisms in each sampling methods among four groups. Scatterplot shows the PCA analysis (a), the PCoA analysis (b), the Beta diversity heatmap (c) and relative abundance of species in phylum level (d) among four groups.
2.2.4. Species distribution
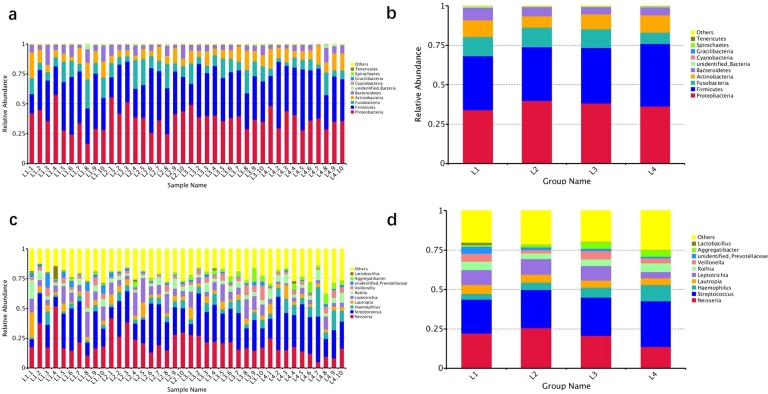
The top 5 dominant bacteria phylum in the 4 groups of samples included Proteobacteria, Firmicutes, Fusobacteria, Actinobacteria, and Bacteroidetes. The top 5 dominant bacteria genus were Neisseria, Streptococcus, Haemophilus, Lautropia, and Leptotrichia spp.
At the phylum level (Fig. 5a, b), the L1 group compared to the L2 group indicated that an increase in the abundance of the Firmicutes, Bacteroidetes, and a decrease in the abundance of the Fusobacteria, Actinobacteria, and Proteobacteria in the patients with dental caries, based on the presence of HSP. A comparison of the L1 group with the L3 group showed an increase in the abundance of Fusobacteria, Bacteroidetes, and Actinobacteria, and a decrease in the abundance of Proteobacteria and Firmicutes in the oral cavity of patients with HSP under the prerequisite condition of having dental caries. L2 group compared with L4 group indicated that, compared with healthy children, an increased abundance of Proteobacteria, Fusobacteria, and Bacteroidetes and a decrease abundance of Firmicutes and Actinobacteria in the oral cavity of patient with HSP. A comparison of the L3 group compared with the L4 group indicated that, compared with healthy children, an increase in the number of Fusobacteria and Proteobacteria and a decrease in the number of Firmicutes, Actinobacteria, and Bacteroidetes in children with dental caries. The abundance of Firmicutes was significantly decreased in the L1 and L2 groups with HSP compared with the L3 and L4 groups without HSP.
Figure 5.
The Species Distribution in each group. Intra- and inter-group distribution of microorganisms in the oral cavity among the four groups of patients at the phylum level (a, b). Intra- and inter-group distribution of microorganisms in the oral cavity among the four groups of patients at the genus level (c, d).
At the genus level (Fig. 5c, d), the L1 group compared to the L2 group indicated that, based on HSP, increased abundance of Lautropia, and decreased abundance of Leptotrichia, Neisseria, Haemophilus, and Streptococcus was present in patients with dental caries. L1 group compared to the L3 group indicated that based on caries, patients with HSP showed increased abundance of Neisseria, Lauteropia, and Leptotrichia, and decreased abundance of Haemophilus and Streptococcus. L2 group compared with the L4 group showed that, compared with healthy children, the presence of HSP may cause an increase in the abundance of Neisseria, Lautropia, and Leptotrichia, and a decrease in the abundance of Streptococcus and Haemophilus. A comparison of the L3 group with the L4 group indicated that compared with healthy children, caries might induce an increase in the abundance of Neisseria, Lautropia, and Leptotrichia, and a decrease in the abundance of Streptococcus and Haemophilus. The largest changes in the abundance of Neisseria and Streptococcus were observed in the L1 and L2 groups with HSP compared with L3 and L4 groups without HSP, with an increase in the abundance of Neisseria and a decrease in the abundance of Streptococcus in two groups with HSP.
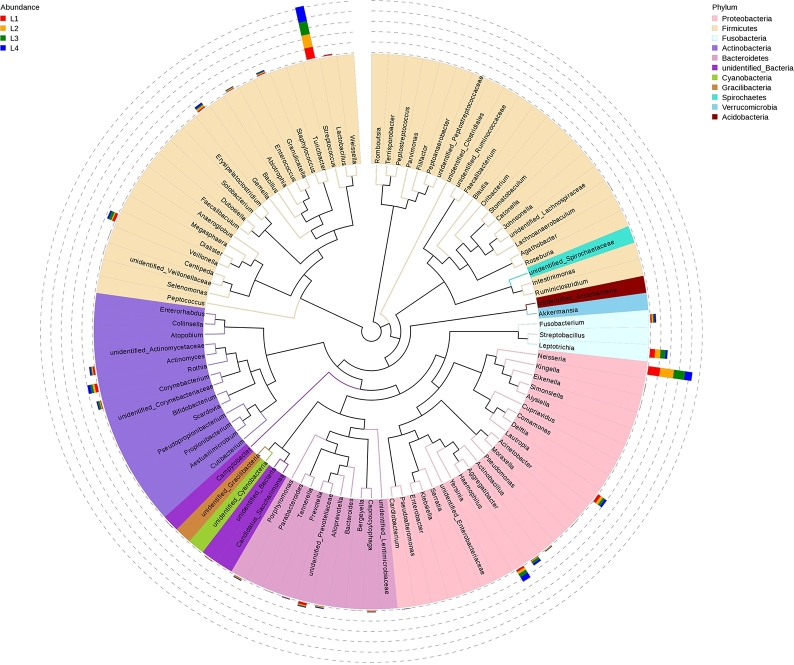
To further investigate the phylogenetic relationships of species at the genus level, representative sequences of the top100 genus were obtained by multiple sequence alignment, as shown in Fig. 6. The top three genus—Neisseria, Streptococcus, and Leptotrichiahad a high abundance distribution in different samples.
Figure 6.
Phylogenetic tree of the top 100 genera in four groups at the genus level. The colors of the branches and sectors indicate their corresponding phylum, and the stacked bar graphs on the outside of the fan ring indicate information on the abundance distribution of the genus in different samples.
2.2.5. Species similarity analysis
The Venn diagram is shown in Fig. 7. A total of 1892 bacteria strains were detected in the 4 groups, 407 of which were common to all 4 groups. These bacteria may constitute the core microorganisms of the oral cavity. The detection of bacterial strains in L1 and L2 groups with HSP was much lower than in L3 and L4 groups without HSP, indicating that the oral flora richness was reduced when HSP was present. Ten bacterial strains were specific to L1 and L2 groups, which might be closely related to the occurrence of HSP, and 23 bacterial strains were specific to L1 and L3 groups, which might be associated with the occurrence of caries.
Figure 7.
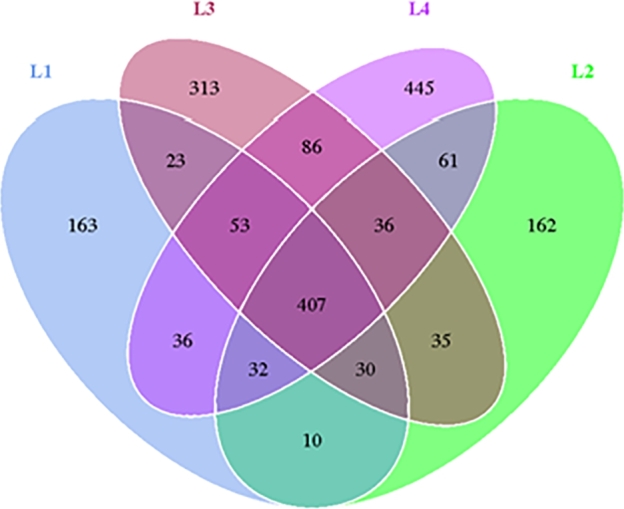
Venn chart of oral microorganisms between different groups.
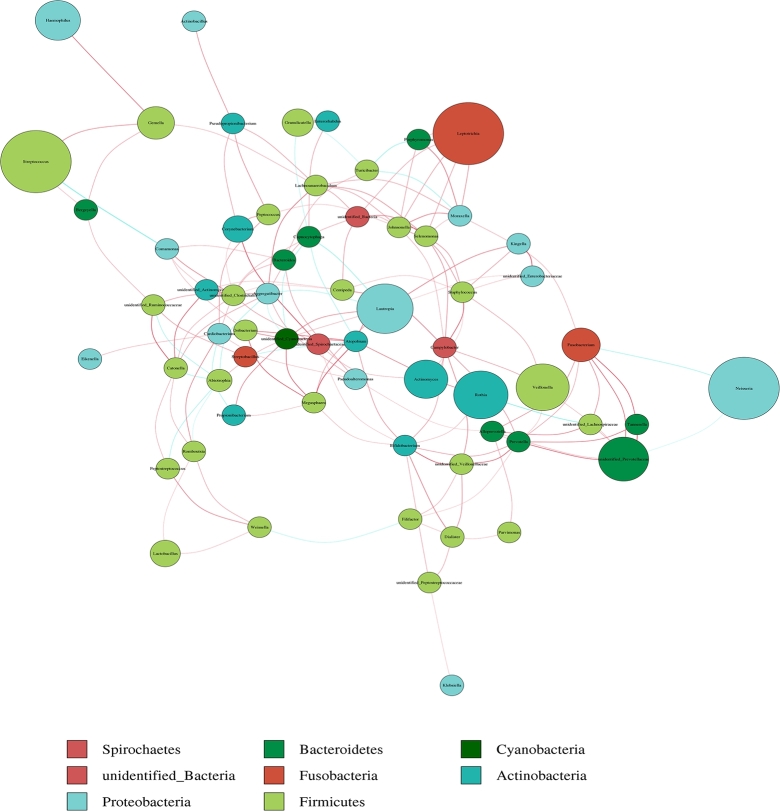
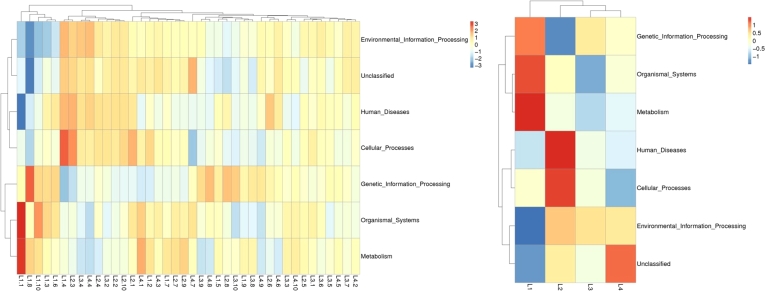
At the same time, since the symbiotic relationships of microorganisms tend to vary across environments, species symbiotic network maps (Fig. 8) allow us to visualize the important role that these dominant microbial may have in maintaining the stability of the microbial community structure and function in that environment. Similar to the previous results, the top three genus—Neisseria, Streptococcus, and Leptotrichia had a high abundance distribution in different samples, Firmicutes, Proteobacteria, and Actinobacteria had the highest number of species, with the existence of intensive communication links between many microorganisms. We selected the top 35 genera in terms of abundance, made heat maps of their functions and their abundance information in each sample, and clustered them at the level of functional differences, finding out that they may have a role in metabolism, human diseases, organismal systems, and cellular processes (Fig. 9).
Figure 8.
Network analysis among oral microorganisms. Different nodes represent different genera, node size represents the average relative abundance of the genus, nodes of the same clade have the same color (as shown in the legend), the thickness of the line between nodes is positively correlated with the absolute value of the correlation coefficient of species interactions (red–positive, blue–negative).
Figure 9.
Cluster analysis of the relative abundance of oral microorganisms' functions intra- and inter-groups. The top 35 functions in terms of abundance and their informative heatmap of abundance in each sample are shown on the left, and the functional difference clustering analysis is shown on the right.
3. Discussion
Oral microbial disorders can cause oral diseases or systemic diseases. In fact, numerous studies have shown a significant correlation between immune system diseases and oral microorganisms [22]. Bacteria are the initiating factor of caries, and a large number of bacteria such as Streptococcus and Neisseria can be detected at the site of caries [23]. As a source of infection, dental caries may cause or aggravate infectious diseases such as infective endocarditis (IE). In the endocardium of patients affected by IE, researchers have found caries-associated Streptococcus mutans and observed that caries treatment helps the recovery of IE [24]. In addition, bacteria at the site of carious lesions stimulate an immune response in the body, potentially exacerbating allergic diseases [25], [26].
HSP is the most common systemic small vessel vasculitis in childhood. Infection may be a predisposing factor for this disease. Also, considering it is a type of autoimmune disease, we pondered whether the development of HSP could be related to oral microorganisms. Our study showed that the caries rates of children with HSP in the 3 and 4-year-old groups were higher than the results reported by the fourth national oral epidemiological survey. The same trend was observed for the Mean dmftin the 3-5 years old group of HSP children. Inoue et al. [19] examined the teeth of 40 children with HSP disease, finding that they had severe oral disorders, especially caries (70%) and periapical infection (5%). Also, the clinical symptoms of those children with HSP were relieved by root canal therapy and antibacterial treatment of the infected lesions. Furthermore, Lai et al. [20] found that the duration of renal damage in children with HSPN increased when they also had dental caries. The incidence of nephritis was also significantly increased when children simultaneously had HSP and dental caries. Moreover, it was also observed that the treatment of dental caries helped the recovery of renal damage in children with HSP.
Similar to previous studies [27], the main genus of oral bacteria was widely present in the four groups of patients in our study; however, the oral microbial richness and diversity differed between the four groups, as did the structure of the flora. Compared to the healthy controls, both groups with HSP had decreased oral microbial richness degree and diversity, which suggested that a disturbing oral microflora accompanies the occurrence of HSP. In the case of oral ecological dysbiosis, the oral pathogenic flora may become dominant and inhibit the growth and reproduction of other bacteria, which is consistent with other studies of immune system diseases [28]. Compared to healthy controls, children with caries had an elevated abundance of the Proteobacteria and decreased abundance of the Firmicutes, which is in line with the findings of Chen et al. [29]. At the phylum level, the Firmicutes were decreased in both groups with HSP compared to both groups without HSP. Firmicutes can produce butyric acid [30], which protects and repairs the mucosa by increasing the resistance of the epithelium allowing tight junction proteins to localize around the cells [31]. In addition, butyric acid induces the development of Treg cells, a reduction in butyric acid that may lead to a decrease in the number of Treg cells, ultimately leading to immune imbalance [32]. This may be one of the etiological mechanisms of HSP. At the genus level, the largest changes in relative abundance between the groups with or without HSP were Streptococcus and Neisseria. Many studies have found that some structures of Streptococcus are similar to body tissues. When they enter the body, the immune system misidentifies the body tissues as Streptococcus, thus inducing an abnormal immune response. This, in turn, increases abnormal immune complexes mediated by immunoglobulin A, which activates complement and deposits in the wall of small blood vessels, thus leading to the development of HSP [33]. In addition, Streptococcus infection can also have a regulatory role by the immune system throughout the process of HSP initiation, pathogenesis, and recurrence [34]. Tsolia et al. [35] previously reported two cases of HSP due to Neisseria invasive meningitis infection. The clinical symptoms of Neisseria infection are often manifested as skin vasculitis and arthralgias, which are similar to those of HSP. This is why it is presumed that Neisseria may be one of the risk factors for the development of HSP.
Alpha diversity analysis revealed that HSP might reduce the richness and diversity of oral microflora. The results of PCA analysis showed that both HSP and caries might cause a decrease in the abundance and diversity of oral microflora compared to healthy children. The Venn diagram showed 10 strains of bacteria specific to the L1 and L2 groups with HSP, which may be closely related to the occurrence of HSP. Further testing of these bacteria is recommended to verify our speculation. Network analysis, just as functional prediction, gives us some direction as to how the oral microbial environment may be associated with developing many diseases.
Genetic network diagrams provide a new perspective for studying community structure and function in complex microbial environments. Since the symbiotic relationships of microorganisms in different environments are completely different, the species symbiosis network diagram can directly show the effects of different environmental factors on microbial adaptations, as well as the dominant species and closely interacting species groups in a certain environment. These dominant species and species groups often play a unique and important role in maintaining the structural and functional stability of microbial communities in the environment [36], [37]. In the present study, in the symbiotic network mapped after the calculation of correlation indices for all the samples, the presence of dense communication links between microorganisms such as Firmicutes, Proteobacteria, Actinobacteria, etc., thus may have played a significant role. It also suggests that they are the core microorganisms in the oral flora. Dysbiosis of oral flora may promote the occurrence and development of local inflammation in the oral cavity, which may lead to the increase of oral epithelial inter-cellular permeability and the destruction of oral mucosal barrier, so that Corynebacterium glabrata or its metabolite lipopolysaccharides can enter into the whole body through the oral mucosal barrier, leading to the occurrence of metabolic endotoxemia [38]. This excessive inflammation may further affect the expression and function of important immune-inflammatory molecules, Also these bacteria are involved in human diseases, cellularprocesses, metabolism and other functions, which will in turn have an impact on the occurrence and development of Henoch-Schönlein purpura to some extent.
The age of the children with HSP included in our study was mainly concentrated around 3-5 years old. Also, as there were a small number of cases in the remaining groups, it is suggested to increase the sample size in the follow-up experiment to continue the study.
4. Methods
4.1. Survey on the oral health status of children with Henoch-Schonlein Purpura (HSP)
Cases diagnosed with HSP at the Department of Pediatrics, General Hospital of Ningxia Medical University between March 2020 and October 2021 were included. (Diagnostic criteria meet EULAR/PRS diagnostic criteria for anaphylactic purpura [11]) Basic information such as name, age, gender, and contact information of the children with HSP was recorded before the examination. At the same time, the oral health conditions of those children were examined and recorded, and the incidence of dental caries and decayed, missing, and filled teeth index (dmfs/DMFS) were calculated and analyzed. The DMFS was used to represent the permanent tooth, and the dmfs were used to represent the primary tooth. The inspectors were 3 dental practitioners (all of whom had dental licenses), who underwent uniform training before the inspection to clarify the inspection content and diagnostic criteria. All 3 inspectors passed the standard consistency test.
4.2. Analysis of oral microbial diversity in children with HSP and children with caries
To better compare and explore the relationships between caries and HSP, we recollected four groups of cases according to the type of disease (10 patients per group): Group L1; patients with both HSP and caries; Group L2: patient with HSP only; Group L3: patients with caries only; Group L4: healthy children (neither HSP nor caries).
The exclusion and inclusion criteria are as follows: In L1 group and L2 group, children were clearly diagnosed HSP and hospitalized in Department of Pediatrics, General Hospital of Ningxia Medical University. The children were all younger than 14 years old, and no other systemic diseases other than HSP were diagnosed. Group L3 and group L4 were children under 14 years old who were treated in Stomatological Hospital, General Hospital of Ningxia Medical University without any diagnosis of systemic diseases. None of the children had received antibiotics, immunomodulators, or immunoglobulins in the 4 weeks prior to sampling. Children with intellectual or physical disabilities who cannot cooperate with the examination, or children or parents who refuse to participate will be excluded. There was no statistically significant difference in the gender and age of the patients included in the above groups.
Plaque samples were collected from children enrolled in the study between 8 and 10 am. Children were not allowed to brush their teeth and gargle and eat or drink water before sample collection. The samples were collected from the tooth surfaces of the right lower deciduous molars/permanent molarsand were sequenced by 16SrDNA high-throughput sequencing. The steps are as follows:
The 16SrDNA sequencing technology was used to detect the bacterial plaque flora, and the sequencing platform was Illumina Mi Seq2×300 bp. The sequencing process: (1) DNA extraction: add 2 mL of plaque suspension into a sterile centrifuge tube, centrifuge at 10 000 r/min (centrifugation radius 10 cm) for 3 min at room temperature, discard the supernatant, and then extract the DNA according to the instructions of the kit, and detect the integrity of the extracted DNA by 2% agarose gel electrophoresis. Perform DNA amplification. (2) Gene construction: 20 ng of DNA was taken after DNA quantification for DNA library construction, including isolation of large DNA fragments, recovery of small DNA fragments, repairing DNA ends, connecting A-joints, connecting the special joints of Illumina Sequencing Kit to both ends of the DNA, magnetic bead screening according to the size of the required DNA fragments, and PCR amplification of the library for probe hybridization capture and sequencing experiments. (3) Capture of genes: the Geneseeq hybridization enrichment probe should be used to enrich and amplify the target gene target of the constructed DNA library, including library hybridization and DNA capture probe, cleaning and recycling of the captured library product, binding of streptavidin magnetic beads to the captured library, cleaning of the magnetic beads captured library, and removal of the non-specific binding library. (4) Sequencing: After library capture, according to the kit operating instructions, Illumina high-throughput sequencing platform up-sampling, in the Flow cell kit to generate DNA clusters, through the synthesis of a single base after the suspension of the cycle of fluorescence detection, synthesis recovery. The 16SrDNA high-throughput test was performed by Novogene, Inc.
5. Data analysis
Library construction using TruSeq® DNA PCR-Free Sample Preparation Kit for library construction. Up-sequencing was performed using NovaSeq6000. Using Uparse software (Uparse v7.0.1001, http://www.drive5.com/uparse/)to cluster all Effective Tags of all samples, the sequences were clustered by default with 97% agreement (Identity) into OTUs (Operational Taxonomic Units). Chao1, Shannon, Simpson, ace indices were calculated using Qiime software (Version 1.9.1), and used R software to conduct the Alpha diversity index inter-group variance analysis; Alpha diversity index inter-group variance analysis would be conducted with and without parametric tests, respectively, and the Tukey test and the wilcox test were chosen. UPGMA sample clustering trees were constructed using Qiime software (Version 1.9.1). PCA, PCoA plots were drawn and analyzed using R software (Version 2.15.3). Beta diversity index intergroup variance analysis was performed using R software with parametric and non-parametric tests, respectively, and the Tukey test and wilcox test were chosen. Based on the species abundance, the correlation coefficient values (Spearman correlation coefficient SCC or Pearson correlation coefficient PCC) were calculated between the genera to obtain the correlation coefficient matrix. Based on the filtered correlation coefficient values, network plotting was performed using graphviz-2.38.0.
6. Study limitations
The study has potential limitations. This was a small sample size single-center study; the experiment was carried out in the selected population of patients in Ningxia Medical University General Hospital Which may limit the generalization of the findings. Meanwhile, our study did not involve the collection of oral health care measures, dietary habits, feeding habits and other information of the included patients, and future studies will need to consider more influential factors.
7. Conclusions
In this study, the children with HSP included in this experiment were mainly 3-5 years old. Except for the incidence of dental caries in the 5 years old group, the incidence of dental caries and dmfs/DMFS in 3- and 4-years old groups were higher than that in the same-age children, as reported by the fourth Chinese Oral Health Epidemiological Survey [21]. The oral health conditions of the children with HSP in the 3-5 years old group were poorer.
The oral microorganisms of children with HSP were examined using 16SrDNA high-throughput sequencing technology. The occurrence of HSP was accompanied by the disorder of oral microorganisms. The reduction of Firmicutes that produced butyric acid may be closely related to the occurrence of HSP. In addition, compared to normal children, the abundance of Neisseria increased and Streptococcus decreased in the oral cavity of children with HSP. These results may guide the etiology and treatment of HSP, but the related mechanisms need further study.
Ethics statement
All experiments were performed in accordance with relevant guidelines and regulations. This study was reviewed and approved by the Ethics Committee of General Hospital of Ningxia Medical University, with the approval number: KYLL-2020-302. All participants/patients (or their proxies/legal guardians) provided informed consent to participate in the study. All participants/patients (or their proxies/legal guardians) provided informed consent for the publication of their anonymised case details and images.
CRediT authorship contribution statement
Rui min Li: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Funding acquisition. Zhe Long: Validation, Investigation, Data curation. Xiao yan Ding: Investigation, Formal analysis. Li Duan: Investigation.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Rui min Li reports financial support was provided by Ningxia Hui Autonomous Region Natural Science Foundation (2020AAC02036). Rui min Li reports financial support was provided by China Oral Health Foundation (A2021-135). Rui min Li reports financial support was provided by Key R & D projects of Ningxia Autonomous Region (2020BEG03029). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by: Natural Science Foundation of Ningxia Province of China (2020AAC02036), Research project of China Oral Health Foundation (A2021-135), Key R & D projects of Ningxia Autonomous Region (2020BEG03029) Gratefully acknowledged.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Data related to this study were uploaded to GSA-human https://bigd.big.ac.cn/gsa-human/ (Submission ID: subHRA 004682).
References
- 1.Zhou W., Zhang D.S. The microbial diversity of oral microorganisms: research progress. China J. Microecol. 2015;27:738–741. [Google Scholar]
- 2.Shi W.Y., Zhou X.D. The interaction of human oral microorganisms. West China J. Stomatol. 2010;28:1–4. [PubMed] [Google Scholar]
- 3.Zhu R., Qin H.L. Progress of research on the association of oral microorganisms with oral diseases, intestinal flora, and intestinal diseases. Shanghai J. Prev. Med. 2020;32:256–261. [Google Scholar]
- 4.Komiya Y., Shimomura Y., Higurashi T., Sugi Y., Rimoto J., Umezawa S., et al. Patients with colorectal cancer have identical strains of Fusobacterium nucleatum in their colorectal cancer and oral cavity. Gut. 2019;68:1335–1337. doi: 10.1136/gutjnl-2018-316661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pushalkar S., Ji X., Li Y., Estilo C., Yegnanarayana R., Singh B., et al. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012;12:144. doi: 10.1186/1471-2180-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominy S.S., Lynch C., Ermini F., Benedyk M., Arczyk A., Konradi A., et al. Porphyromonasgingivalis in Alzheimer's disease brains: evidence for disease causation and treatment with small molecule inhibitors. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Bars P., Matamoros S., Montassier E., Le Vacon F., Potel G., Soueidan A., et al. The oral cavity microbiota: between health, oral disease, and cancers of the aerodigestive tract. Can. J. Microbiol. 2017;63:475–492. doi: 10.1139/cjm-2016-0603. [DOI] [PubMed] [Google Scholar]
- 8.Torres P.J., Fletcher E.M., Gibbons S.M., Bouvet M., Doran K.S., Kelley S.T. Characterization of the salivary microbiome in patients with pancreatic cancer. PeerJ. 2015;3 doi: 10.7717/peerj.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He J.Z., Xu X., Zhou X.D. Advances in oral microbiology and systemic health research. Microbes Infect. 2017;12:139–145. [Google Scholar]
- 10.Leung A.K.C., Barankin B., Leong K.F. Henoch-Schönlein purpura in children: an updated review. Curr. Pediatr. Rev. 2020;16:265–276. doi: 10.2174/1573396316666200508104708. [DOI] [PubMed] [Google Scholar]
- 11.Ozen S., Pistorio A., Iusan S.M., et al. EULAR/PRINTO/PRES criteria for Henooch-Schonlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann. Rheum. Dis. 2010;69(69):798–806. doi: 10.1136/ard.2009.116657. [DOI] [PubMed] [Google Scholar]
- 12.Kang Y., Park J.S., Ha Y.J., et al. Differences in clinical manifestations and outcomes between adult and child patients with Henoch-Schonlein purpura. J. Korean Med. Sci. 2014;29:198–203. doi: 10.3346/jkms.2014.29.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan Y.X., Ye Q., Shao W.X., et al. Relationship between immune parameters and organ involvement in children with Henoch-Schonlein purpura. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purevdorj N., Mu Y., Gu Y., et al. Clinical significance of the serum biomarker index detection in children with Henoch-Schonlein purpura. Clin. Biochem. 2018;52:167–170. doi: 10.1016/j.clinbiochem.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Al-Sheyyab M., Batieha A., el-Shanti H., et al. Henoch-Schonlein purpura and streptococcal infection: a prospective case-control study. Ann. Trop. Paediatr. 1999;19:253–255. doi: 10.1080/02724939992329. [DOI] [PubMed] [Google Scholar]
- 16.Masuda M., Nakanishi K., Yoshizawa N., et al. Group A streptococcal antigen in the glomeruli of children with Henoch-Schonlein nephritis. Am. J. Kidney Dis. 2003;41:366–370. doi: 10.1053/ajkd.2003.50045. [DOI] [PubMed] [Google Scholar]
- 17.Zhu J.F. Study on the correlation between pediatric streptococcal infection and the pathogenesis of Henoch-Schönlein Purpura. Jilin Med. J. 2013;34:4691–4692. [Google Scholar]
- 18.Chen P., Xiao F.L. Progress of research on the etiology and pathogenesis of Henoch-Schönlein Purpura. J. Pract. Dermatol. 2011;4:93–95. [Google Scholar]
- 19.Inoue C.N., Matsutani S., Ishidoya M., Homma R., Chiba Y., Nagasaka T. Periodontal and ENT therapy in the treatment of pediatric Henoch-Schönlein purpura and IgA nephropathy. Adv. Otorhinolaryngol. 2011;72:53–56. doi: 10.1159/000324605. [DOI] [PubMed] [Google Scholar]
- 20.Lai D.B. Effect of caries on renal damage of Henoch-Schönlein Purpura. J. Pract. Med. 2003;19:1023–1024. [Google Scholar]
- 21.http://www.gov.cn/xinwen/2017-09-20/content5226224.htru The fourth national oral health epidemiological survey, National Health and Family Planning Commission of the People's Republic of China.
- 22.Vilela E.M., Bastos J.A., Fernandes N., Ferreira A.P., Chaouban A., Bastos M.G. Treatment of chronic periodontitis decreases serum prohepcidin levels in patients with chronic kidney disease. Clin. 2011;66:657–662. doi: 10.1590/S1807-59322011000400022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu L., Chen X., Wang Y., Jiang W., Wang S., Ling Z.X., et al. Dynamic alterations in salivary mi-crobiota related to dental caries and age in preschool children with deciduous dentition: a 2-year follow-up study. Front. Physiol. 2018;9 doi: 10.3389/fphys.2018.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen D., Hao Y.Q. Relationship between oral microorganisms, oral treatment and infective endocarditis. J. Mod. Stomatol. 2009;23:423–426. [Google Scholar]
- 25.Larsen J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151:363–374. doi: 10.1111/imm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hitchon C.A., Chandad F., Ferucci E.D., Willemze A., Facsinay A.L., Woude D., et al. Antibodies to Porphyromonasgingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J. Rheumatol. 2010;37:1105–1112. doi: 10.3899/jrheum.091323. [DOI] [PubMed] [Google Scholar]
- 27.Machado D., Castro J., Martinez J., Nogueira C., Cerca N. Prevalence of bacterial vaginosis in Portuguese pregnant women and vaginal colonization by Gardnerella vaginalis. PeerJ. 2017;5:3748–3750. doi: 10.7717/peerj.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falsetta M.L., Klein M.I., Colonne P.M., Scott K., Gregoire S., Pai C.H., et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 2014;82:1968–1981. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen B., Wang J.W., Wang Y., Zhang J.M., Zhao C.Y., Shen N., et al. Oral microbiota dysbiosis and its association with Henoch-Schönlein Purpura in children. Int. Immunopharmacol. 2018;65:295–302. doi: 10.1016/j.intimp.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Chen T.T., Shi Y., Wang X.L., Wang X., Meng F.J., Yang S.G., et al. Hingh throughput sequencing analyses of oral microbial diversity in healthy people and patients with dental caries and periodontal disease. Mol. Med. Rep. 2017;16:127–132. doi: 10.3892/mmr.2017.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan Q.X., Wang C.M., Sheng G.Y. Role of Th17 cells and interleukin-17 in the pathogenesis of Henoch-Schonlein purpura in children. J. China Pract. Diagn. Theat. 2010;24:1089–1090. [Google Scholar]
- 32.Sean M.N., Tara H. Short chain fatty acids in the colon and peripheral tissues: a focus on butyrate, colon cancer, obesity and insulin resistance. Nutr. 2017;9:13–48. doi: 10.3390/nu9121348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu M., Hamaguchi Y., Matsushita T., Sakakibara Y., Yachie A. Sequentially appearing erythema nodosum, erythema multiforme and Henoch-Schonlein purpura in a patient with Mycoplasma pneumoniae infection: a case report. J. Med. Case Rep. 2012;6:398. doi: 10.1186/1752-1947-6-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao J., Nian L., Kwok L.Y., Sun T., Zhao J. Reduction in fecal microbiota diversity and short-chain fatty acid producers in Methicillin-resistant Staphylococcus aureus infected individuals as revealed by PacBio single molecule, real-time sequencing technology. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36:1463–1472. doi: 10.1007/s10096-017-2955-2. [DOI] [PubMed] [Google Scholar]
- 35.Tsolia M.N., Fretzayas A., Georgouli H., Tzanakaki G., Fessatou S., Liapi G., et al. Invasive meningococcal disease presenting as Henoch-Schönlein purpura. Eur. J. Clin. Microbiol. Infect. Dis. 2004;23:776–779. doi: 10.1007/s10096-004-1203-8. [DOI] [PubMed] [Google Scholar]
- 36.Jiao S., Liu Z.S. Bacterial communities in oil contaminated soils: biogeography and co- occurrence patterns. Soil Biol. Biochem. 2016;98 [Google Scholar]
- 37.Qin J.J., Li Y.R., Cai Z.M., Li S.H., Zhu J.F., Zhang F., et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 38.Nagpal R., Yamashiro Y., Izumi Y. The two-way association of periodontal infection with systemic disorders: an overview. Mediat. Inflamm. 2015;793898 doi: 10.1155/2015/793898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Data related to this study were uploaded to GSA-human https://bigd.big.ac.cn/gsa-human/ (Submission ID: subHRA 004682).