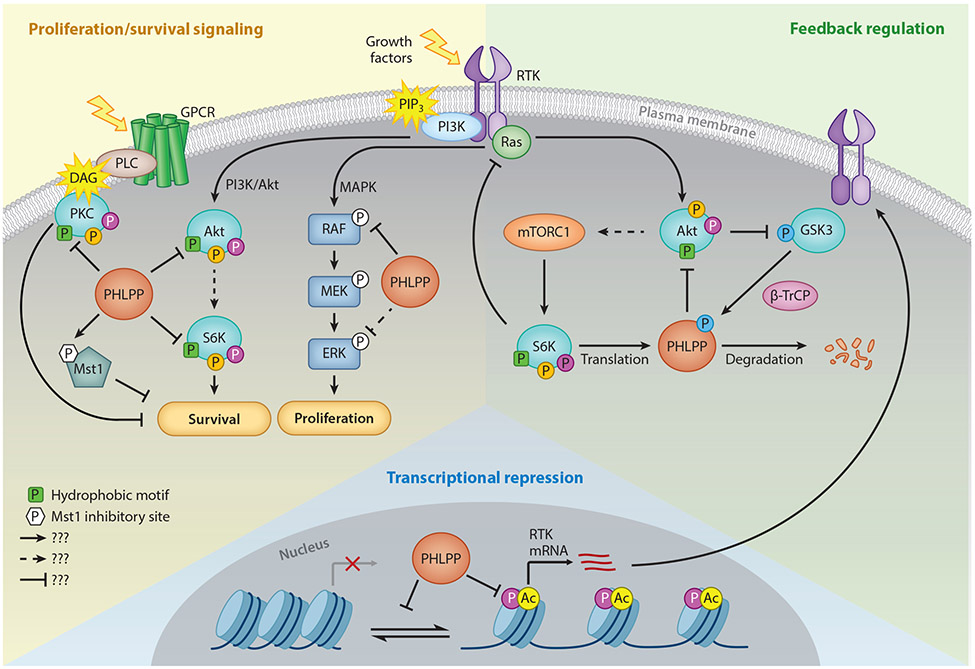
Figure 2. PHLPP Function in Growth Factor Signaling.
Schematic showing some of the substrates and signaling pathways regulated by PHLPP. In general, PHLPP suppresses proliferative and survival pathways through direct dephosphorylation of Akt, S6K1, Mst1, and Raf (red sector). PHLPP dephosphorylates the hydrophobic motif (green square with P) of Akt and S6K1 to inactivate them, while PHLPP activates the pro-apoptotic kinase, Mst1, via removal of an inhibitory phosphorylation (white hexagon with P). PHLPP also dephosphorylates PKC isozymes on the hydrophobic motif, which destabilizes them and reduces their cellular levels. Filled in circles on Akt, S6K1, and PKC denote other key phosphorylation sites, including the PDK-1 site on the activation loop (magenta circle) and mTORC2-regulated turn motif (orange circle). In addition, PHLPP suppresses the transcription and hence steady-state protein levels of RTKs such as the EGFR, thus dampening PI3K survival and ERK proliferative pathways, by an epigenetic mechanism (blue sector). PHLPP itself is under feedback regulation by components of the PI3K pathway (tan sector). High Akt activity increases PHLPP protein levels by suppressing GSK3β-dependent degradation. Furthermore, high mTORC1 activity increases S6K1-dependent translation of PHLPP (left). Moreover, S6K1 activity also activates a negative feedback loop that suppresses Akt activity.