Figure 3. Metabolic regulation of BTN3A.
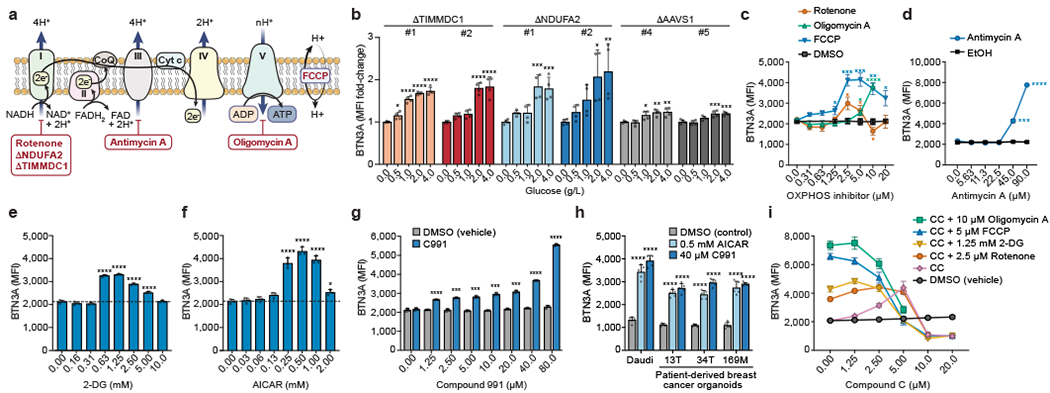
(a) Schematic of OXPHOS, inhibitor targets, and genetic KOs. (b) Surface BTN3A MFI in Daudi-Cas9 KOs cultured in different glucose concentrations for 3 days in RPMI (no glucose, no pyruvate). Normalized to cells grown without glucose (0 g/L). (c, d) Surface BTN3A MFI in WT Daudi-Cas9 cells cultured with vehicles (DMSO, ethanol) or OXPHOS inhibitors of (c) Complex I (rotenone), Complex V (oligomycin A), mitochondrial membrane potential (FCCP), and (d) Complex III (antimycin A) for 72 hours in complete RPMI. (e-g) Surface BTN3A MFI in WT Daudi-Cas9 cells cultured with (e) 2-DG, (f) AICAR, and (g) Compound 991 (C991) or equivalent amount of DMSO (vehicle) for 72 hours in complete RPMI. (h) Surface BTN3A MFI in patient-derived breast cancer organoids and Daudi cells cultured for 3 days with pamidronate and AICAR, C991, or DMSO. (i) Surface BTN3A MFI in WT Daudi-Cas9 cells co-treated with an OXPHOS/glycolysis inhibitor and increasing amounts of Compound C (CC, AMPK inhibitor). (b) n=4 per condition, data combined from two independent experiments, each individually normalized. (c) n=4 per condition, data combined from two independent experiments. (d) n=3 per condition, representative data from one of two experiments. (e, f) n=3 per condition, representative data from one of three independent experiments. (g) n=3 per condition, representative data from one of two independent experiments. (h) n=5, data combined from two independent experiments. (i) n=3 per condition, representative data from one of three independent experiments. (b, e, f, h) One-way ANOVA comparison to the zero or control treatment condition with Dunnett’s multiple comparisons test. (c) Two-tailed unpaired Student’s t test with FDR adjustment for the tested concentrations (1.25-20 μM). (d, g) Two-tailed unpaired Student’s t test with Bonferroni correction. (b-i) Mean ± SD. p<0.0001 (****), p<0.001 (***), p<0.01 (**), p<0.05 (*).
