Abstract
Background
Antithrombotic therapy is crucial for secondary prevention of cardiovascular disease (CVD), but women with CVD may face increased bleeding complications post-percutaneous coronary intervention (PCI) under antithrombotic therapy. However, women are often underrepresented in clinical trials in this field, so evidence for sex-specific recommendations is lacking.
Methods and Results
A search on PubMed was conducted for English-language articles addressing bleeding complications and antithrombotic therapy in women. Despite women potentially showing higher baseline platelet responsiveness than men, the clinical implications remain unclear. Concerning antiplatelet therapy post-PCI, although women have an elevated bleeding risk in the acute phase, no sex differences were observed in the chronic phase. However, women require specific considerations for factors such as age, renal function, and weight when determining the dose and duration of antiplatelet therapy. Regarding anticoagulation post-PCI, direct oral anticoagulants may pose a lower bleeding risk in women compared with warfarin. Concerning triple antithrombotic therapy (TAT) post-PCI for patients with atrial fibrillation, there is a lack of evidence on whether sex differences should be considered in the duration and regimen of TAT.
Conclusions
Recent findings on sex differences in post-PCI bleeding complications did not provide enough evidence to recommend specific therapies for women. Further studies are needed to address this gap and recommend optimal antithrombotic therapy post-PCI for women.
Key Words: Anticoagulants, Antiplatelet therapy, Cardiovascular disease, Percutaneous coronary intervention (PCI), Sex
It is well-established that antithrombotic therapy post-percutaneous coronary intervention (PCI) plays a crucial role in the secondary prevention of cardiovascular disease (CVD).1–3 However, there is a concern about the potentially higher risk of bleeding among women with CVD under antithrombotic therapy.
Although large randomized controlled trials (RCTs) have investigated the efficacy and safety of various antithrombotic agents in CVD patients, women are underrepresented in those trials, constituting only around a maximum 30%.4 The factors contributing to this underrepresentation include (1) women’s reluctance to participate in clinical trials due to a more serious perception of a greater risk of harm from trial participation and potentially lower socioeconomic status compared with men,5,6 (2) the presence of specific exclusion criteria, such as older age and childbearing potential,4 and (3) potential researchers bias, leading to the exclusion of women with CVD in trials due to atypical pathology, comorbidities, and older age.
While numerous studies exploring sex differences in bleeding complications and clinical outcomes after PCI, there is a shortage of comprehensive and consistent data on the impact of antithrombotic agents on women.
Consequently, current guidelines predominantly rely on data derived from men, resulting in a lack of sex-specific recommendations for the use of antithrombotic agents.1–3 The causes of acute and chronic bleeding after PCI may vary between the sexes,7,8 and there is limited in-depth discussion on whether bleeding complications after PCI are genuinely more prevalent in women.
In this review, our objective was to analyze the clinical evidence concerning potential bleeding risks based on sex, and identify discrepancies in indications for oral antithrombotic therapy.
Methods
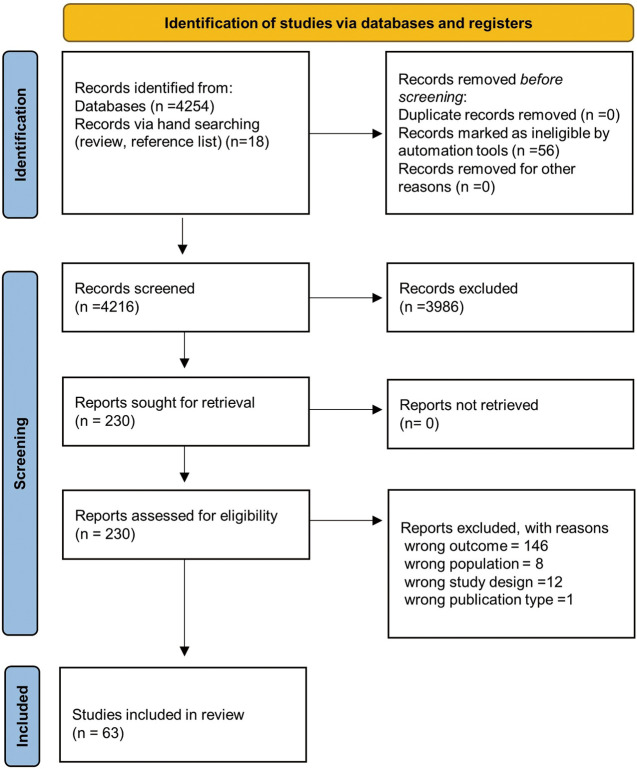
This review paper is based on a comprehensive review of available studies and Figure 1 demonstrates the selection process. We performed English-language searches in PubMed for the period January 1, 2010, to November 3, 2022 using the following keywords: percutaneous coronary intervention, acute coronary syndrome, myocardial infarction, clopidogrel, prasugrel, ticagrelor, dabigatran, edoxaban, apixaban, rivaroxaban, sex, female, bleed and hemorrhage (Supplementary Appendix 1, Supplementary Table). After identifying all articles, we then reviewed the references from appropriate articles to identify additional references for this review. One investigator (Y.N.) screened titles and abstracts for all articles and identified studies as potentially appropriate for inclusion. We subsequently reviewed the full text of these studies to make a final decision on their appropriateness for inclusion.
Figure 1.
Preferred Reporting Items for Reviews and Meta-Analyses (PRISMA) diagram of the articles included in the analysis.
Results
Platelet Reactivity
A total of 7 studies identified disparities in platelet reactivity between the sexes.9–15 Women are considered to have higher baseline platelet counts, increased fibrinogen binding to platelets, and more pronounced activation through interactions with adenosine 5´-diphosphate, collagen, and other mediators.16–18 Additionally, women exhibit higher levels of inflammatory markers, including C-reactive protein, leukocyte count, and P-selectin expression, together with elevated concentrations of membrane microparticles actively participating in inflammatory processes.17 These findings suggest women show higher platelet reactivity, given the crucial role of activated platelets in mediating the inflammatory response.16 However, in a prospective study evaluating sex differences in platelet activity in patients administered 3 platelet inhibitors, women had a higher rate of in-hospital bleeding complications compared with men, but there were no differences in platelet aggregation using the 3 different agonists, reflecting the treatment effects of GPIIb/IIIa inhibitors, clopidogrel, and aspirin.19 The results were similar in both the acute and chronic phases.
Although an association between high platelet reactivity (HTPR) during antiplatelet therapy and increased risk of ischemic events such as cardiovascular death, nonfatal myocardial infarction, stent thrombosis, and ischemic stroke has been observed,20–22 conflicting reports exist regarding the association between HTPR during antiplatelet therapy and sex. A total of 3 reports showed that HTPR during antiplatelet therapy tended to be more pronounced in women,11,12,15 but 4 studies showed comparable platelet reactivity to aspirin and P2Y12 inhibitors during dual antiplatelet therapy (DAPT) after PCI.19,23–25 In addition, a meta-analysis evaluating sex differences in the cardiovascular efficacy of clopidogrel showed no sex differences in either platelet reactivity or therapeutic efficacy.26,27
Taken together, the findings suggest platelet reactivity at baseline is higher in women than in men, but conflicting data have been reported regarding platelet reactivity to aspirin and P2Y12 inhibitors, and the clinical effect of sex differences in platelet reactivity is still inconclusive.9 Additional research is needed to determine whether sex differences in platelet reactivity can be alleviated with novel antiplatelet agents or dosage adjustments, and whether these interventions indeed have an obvious effect on clinically significant outcomes.
Bleeding Risk in Women With Antiplatelet Therapy
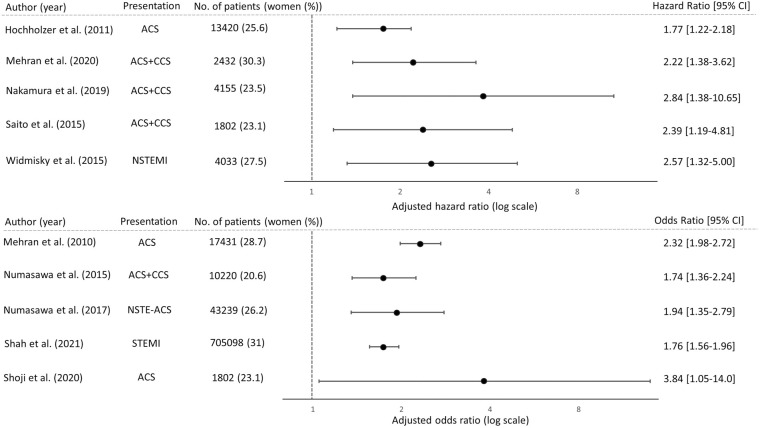
The occurrence of bleeding following PCI under antiplatelet therapy should be evaluated in both the acute and chronic phases. Furthermore, the assessment of risk is refined based on the presence of acute coronary syndrome (ACS) and chronic coronary syndrome (CCS). Research from Europe and the USA has identified women as having a higher risk of in-hospital and short-term bleeding after PCI among patients with ACS (hazard ratio [HR]=1.77–2.57) (Figure 2).28–32 Several studies conducted in Japan, including the PRASFIT-ACS trial,33 PRASFIT-Practice I and II,34,35 JCD-KiCS registry,36 and J-PCI registry,37 have further supported these findings: women exhibited an increased risk of acute bleeding after PCI for ACS (odds ratio [OR]=1.94–3.84) (Figure 2). This sex-related risk extends beyond ACS patients, as observed in a subanalysis of the LEADERS FREE trial,7 PRASFIT-Practice II38 and JCD39 involving CCS patients (HR=2.22 and OR=1.74–3.84) (Figure 2). The main factor contributing to the elevated rate of bleeding in women in the acute phase after PCI for both ACS and CCS is bleeding at the vascular puncture site. The rate of bleeding varies according to the puncture site. Transradial intervention (TRI) reduced bleeding events by up to one-third compared with transfemoral intervention (TFI) in both men and women.7,33,39–41
Figure 2.
Sex-based differences in acute-phase bleeding post-PCI under antithrombotic therapy. Forest plots show that female sex is an independent risk factor in acute-phase bleeding after PCI under antithrombotic therapy. Horizontal lines represent 95% confidence intervals (CI) and circles represent hazard ratios or odds ratios. ACS, acute coronary syndrome; CCS, chronic coronary syndrome; NSTE-ACS, non-ST-elevation-acute coronary syndrome; NSTEMI, non-ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
In contrast, during the chronic phase following PCI, the majority of the studies conducted outside of Japan7,29,42–56 did not identify women as a significant risk factor for bleeding complications in either ACS or CCS patients. Despite East Asians being considered to have an elevated tendency for antiplatelet-induced bleeding, women were also not an independent bleeding factor in the chronic phase in the Korean KAMIR-NIH study52 of both post ACS and CCS patients. Similarly the Japanese PRASFIT-Practice II study revealed that women were not an independent risk factor for bleeding after 31 days within tan observation period of 1 year.35 The CREDO-Kyoto thrombotic and hemorrhagic risk score, developed to predict long-term thrombotic and bleeding events in Japan, does not include women as a specific risk factor.40 In contrast to these findings, 2-year follow-up of the Japanese JCD-KiCS registry found that women remained an independent bleeding risk factor even after 2 years.8 However, that study did not provide details of DAPT duration after PCI, and the implantation of drug-eluting stents (DES) was notably more prevalent in women (58.4% vs. 53.1%) compared with bare metal stents.
Attention should be directed towards age-related considerations in CVD among East Asians. A study of patients aged £55 years in an overseas trial revealed that younger women (24%) had a significantly higher risk of 1-year major adverse cardiovascular events and bleeding than men, although female sex was not an independent factor.57 Conversely, in a Japanese study,58 the percentage of female ischemic heart disease (IHD) patients under 55 years of age was exceedingly small (7.8%) compared with overseas cohorts. However, the prevalence of background factors such as anemia, hemodialysis, and cancer were significantly higher in women than men, all of which are thrombotic and bleeding risks to be considered. There is limited data on long-term bleeding risk related to young women in Japan, and future evidence needs to be accumulated.
Although discrepancies exist in the definition of bleeding complications and the duration of DAPT across various trials, studies have consistently indicated that factors such as advanced age, chronic kidney disease (CKD), and low body weight can account for chronic-phase bleeding in women under antithrombotic therapy after PCI. Consequently, those studies do not consider female sex as an independent factor for chronic-phase bleeding.7,29,35,42–52,55 However, in the future there remains a need for long-term data to integrate P2Y12 inhibitors with the recommended duration of DAPT outlined in recent guidelines.
Data derived from meta-analyses, RCTs, and registries investigating sex differences during the acute and chronic phases of bleeding complications post-PCI are summarized in the Table.
Table.
Sex Differences During (A) Acute Phase and (B) Chronic Phase in Bleeding Complications Post-PCI
| (A) Authors, year, country |
Name of study | Total patients |
No. of women (%) |
Presentation | Topics | Bleeding outcome | Female sex as an independent risk factor of bleeding |
OR or HR [95% CI] |
|---|---|---|---|---|---|---|---|---|
| Hochholzer et al (2011) Multi-country29 |
TRITON-TIMI 38 | 13,420 | 25.6 | ACS | Prasugrel (60 mg LD, 10 mg/day maintenance dose) vs. clopidogrel (300 mg LD, 75 mg/day maintenance dose) |
TIMI major or minor bleeding (instrumented, traumatic and spontaneous) in hospital period and the follow-up period of the trial (6–15 months) |
Yes | HR 1.77 [1.22–2.18]* *Majority (73%) of serious bleeding events occurred within the first 3 days |
| Hess et al (2014) USA†1 |
TRANSLATE-ACS | 6,218 | 27.5 | STEMI or NSTEMI |
ADP-receptor inhibitor within the first 12 months after AMI |
1-year risk of bleeding according to GUSTO and BARC definitions including patient-reported bleeding not brought to clinical attention |
Yes | GUSTO; HR 1.32 [1.06–1.64]* GUSTO moderate or severe; HR 1.63 [1.19–2.24]* *Majority of GUSTO bleeding events observed early after PCI |
| Mehran et al (2010) Multi-country30 |
ACUITY + HORIZONS-AMI |
17,421 | 28.7 | ACS | Development of a practical risk score to predict the major bleeding |
Non-CABG related major bleeding within 30 days |
Yes | OR 2.32 [1.98–2.72] |
| Mehran et al (2020) Multi-country7 |
LEADERS FREE | 2,432 | 30.3 | ACS+CCS | BMS vs. polymer-free, biolimus A9-eluting drug-coated stent with 1-month DAPT |
BARC 3 to 5 major bleeding within 30 days and 60 days |
Yes | HR 2.22 [1.38–3.62] within 30 days HR 2.22 [1.42–3.47] within 60 days |
| Vascular access site major bleeding |
Yes | Unadjusted HR 4.65 [1.99–10.87] |
||||||
| Nakamura et al (2018) Japan34 |
PRASFIT-Practice I | 732 | 23.5 | ACS | Low-dose prasugrel (LD/maintenance dose, 20/3.75 mg) vs. standard-dose clopidogrel administration |
TIMI major and minor bleeding (64.9±73.8 days) |
Yes | NR |
| Nakamura et al (2019) Japan35 |
PRASFIT-Practice II | 4,155 | 23.5 | ACS+CCS | Low-dose prasugrel (LD/maintenance dose, 20/3.75 mg) vs. standard-dose clopidogrel administration |
TIMI major or minor bleeding within 30 days |
Yes | HR 3.84 [1.38–10.65] |
| Numasawa et al (2015) Japan39 |
JCD | 10,220 | 20.6 | ACS+CCS | Examination of sex differences in in-hospital clinical outcomes after PCI |
Those requiring blood transfusion, prolonged hospital stay, or showing a decrease in hemoglobin >3.0 g/dL |
Yes | OR 1.74 [1.36–2.24] |
| Numasawa et al (2017) Japan37 |
Japanese Nationwide Registry |
43,239 | 26.2 | NSTE-ACS | Investigation of sex-related differences in patients with NSTE-ACS who underwent PCI |
In-hospital bleeding (requiring blood transfusion, including access-site and non-access-site bleeding) |
Yes | OR 1.94 [1.35–2.79] |
| Ohya et al (2018) Japan59 |
Single-center cohort | 992 | 25 | ACS | Very low maintenance dose of prasugrel 2.5 mg in HBR patients vs. low dose 3.5 mg |
In-hospital BARC 3 and 5 major bleeding |
No | NR |
| Saito et al (2015) Japan33 |
PRASFIT Trial | 1,802 | 25.3 | ACS+CCS | Low-dose prasugrel (LD/maintenance dose, 20/3.75 mg) vs. standard- dose clopidogrel administration |
Periprocedural TIMI major and minor bleeding within 3 days |
Yes (ACS) | HR 2.39 [1.19–4.81] |
| Periprocedural TIMI major and minor bleeding within 3 days |
No (Elective) | NR | ||||||
| Shah et al (2021) Multi-country31 |
Global meta-analysis of 56 studies |
705,098 | 31 | STEMI | Evaluation of sex-based discrepancies in clinical outcomes and identifying primary driving factors |
Definition varied by study, generally included bleeding requiring transfusion or repeat procedure |
Yes | OR 1.74 [1.56–1.96] |
| Shoji et al (2020) Japan36 |
JCD-KiCS registry | 1,802 | 23.1 | ACS | Low-dose prasugrel vs. standard-dose clopidogrel administration |
TIMI major or minor bleeding within 72 h after PCI |
Yes | OR 3.84 [1.05–14.0] |
| Simonsson et al (2019) Sweden32 |
SWEDEHEART registry |
97,597 | 35.1 | ACS | Development and validation of a new in-hospital bleeding risk score |
In-hospital non-CABG major bleeding defined as fatal, intracranial or bleeding requiring blood transfusion or surgery (including endoscopic and vascular intervention) |
Yes | NR |
| Venetsanos et al (2017)†2 Multi-country |
ATLANTIC trial | 1,862 | 20 | STEMI | Prehospital vs. in-hospital administration of 180 mg ticagrelor |
TIMI or BARC bleeding at 30 days |
No | TIMI major; HR 1.28 [0.47–3.48] BARC type 3–5; HR 1.45 [0.72–2.91] |
| Widimsky et al (2015) Multi-country28 |
ACCOAST | 4,033 | 27.5 | NSTEMI | (A) 30 mg prasugrel LD followed by CAG with an additional 30 mg prasugrel at the time of PCI or (B) placebo LD followed by 60 mg prasugrel at the time of PCI |
TIMI major bleeding through 7 days |
Yes | HR 2.57 [1.32–5.00] |
|
(B) Authors, year, country |
Name of study |
Total patients |
No. of women (%) |
Presentation | Topics | Bleeding outcome |
Female sex as an independent risk factor of bleeding |
|
| Baber et al (2016) USA and Europe50 |
PARIS (External validation of each score was performed in the ADAPT-DES registry) |
4,190 | 25.5 | ACS+CCS | Development of risk scores of major bleeding |
BARC 2 or 5 bleeding within 2 years |
No | |
| Chichareon et al (2020) Multi-country45 |
GLOBAL LEADERS | 15,968 | 23.3 | ACS+CCS | 1-month DAPT+23-month ticagrelor monotherapy vs. 12-month DAPT+12-month aspirin monotherapy after PCI |
BARC 3 or 5 bleeding at 1 year and 2 years |
No | |
| Généreux et al (2015) USA and Europe55 |
ADAPT-DES | 8,582 | 25.9 | ACS+CCS | Incidence, predictors, and prognostic impact of post- discharge bleeding after PCI with DES |
TIMI major or minor bleed; GUSTO severe or moderate bleed; ACUITY major bleed at (<30 days), late (30 days to <1 year), or very late (1–2 years) |
No | |
| Grodecki et al (2018) Multi-country47 |
BleeMACS | 13,727 | 23 | ACS | Post-discharge bleeding among patients on DAPT after ACS |
In-hospital bleeding defined as any TIMI major or minor bleeding, or any GUSTO moderate or severe bleeding, or any BARC 3 bleeding |
No | |
| Hess et al (2014)†1 US |
TRANSLATE-ACS | 6,218 | 27.5 | STEMI or NSTEMI |
ADP-receptor inhibitor within the first 12 months after AMI |
1-year risk of bleeding according to GUSTO and BARC definitions including patient-reported bleeding not brought to clinical attention |
Yes BARC 1; IRR 1.42 [1.26–1.70] BARC 2; IRR 1.72 [1.36–2.14] No BARC ≥3: IRR 1.14 [0.75–1.75] |
|
| Husted et al (2014) Multi-country51 |
PLATO | 18,624 | 28.3 | ACS | Ticagrelor vs. clopidogrel | Non-CABG-related study criteria major bleeding at 7 days, 7–240 days, after day 240 |
No | |
| Kodaira et al (2021) Japan8 |
JCD-KiCS registry | 2,494 | 22 | ACS | Investigation of the differences between sexes for long-term bleeding complication requiring readmission in East Asia |
Any bleeding event requiring readmission during 2-year follow-up |
Yes HR 1.826–1.895 [1.107–3.093] |
|
| Lee et al (2018) Multi-country43 |
CURE, COMMIT, CLARITY-TIMI 28, TRITON-TIMI 38, PLATO, CHANCE, TRILOGY ACS, SPS3 and SOCRATES |
109,570 | 30 | ACS | Newer P2Y12 inhibitors (ticagrelor and prasugrel) vs. clopidogrel |
Defined by the individual studies that used either TIMI or GUSTO, or trial specific criteria |
No | |
| Lee et al (2014) Korea53 |
DES LATE | 5,045 | 30.7 | ACS+CCS | 12-month DAPT after DES implantation followed by aspirin monotherapy vs. further 24-month DAPT |
TIMI major bleeding through 48 months |
No | |
| Lee et al (2018) Korea52 |
KAMIR-NIH | 13,104 | 24.1 | AMI | 12-month DAPT after DES implantation with aspirin and clopidogrel (75 mg/day), ticagrelor (90 mg twice daily) or prasugrel (10 mg/day) |
TIMI major and minor bleeding at 1 year after PCI |
No | |
| Matteau et al (2015) Multi-country56 |
PROTECT + PROTECT US |
9,410 | 27 | ACS+CCS | Predictor of bleeding and ischemic events beyond 1 year |
GUSTO moderate/severe bleeding (median follow-up duration was 4.1 years) |
No | |
| Mehran et al (2020) Multi-country7 |
LEADERS FREE | 2,432 | 30 | ACS+CCS | BMS vs. polymer-free, biolimus A9-eluting drug-coated stent with 1-month DAPT |
Major bleeding (BARC 3–5) and major or minor bleeding (BARC 2–5) through 780 days |
No | |
| Nakamura et al (2019) Japan38 |
PRASFIT-Practice II | 4,155 | 23.5 | ACS+CCS | Low-dose prasugrel (LD/maintenance dose, 20/3.75 mg) vs. standard- dose clopidogrel administration |
TIMI major or minor bleeding within 1 year (after 31 days) |
No | |
| Natsuaki et al (2018) Japan40 |
CREDO-Kyoto registry cohort 2 vs. RESET and NEXT |
9,447 | 25 | ACS+CCS | Development of CREDO-Kyoto thrombotic and bleeding risk scores |
GUSTO moderate or severe bleeding through 3 years excluding in-hospital bleeding |
No | |
| Sawaya et al (2017) Multi-country48 |
EXCELLENT, OPTIMIZE, PRODIGY, RESET, SECURITY and ITALIC PLUS |
11,473 | 30 | ACS+CCS | Short vs. long-term DAPT after DES implantation |
TIMI bleeding in 4 trials, the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events (REPLACE) criteria and BARC bleeding at 1 year |
No | |
| Schreuder et al (2020) Multi-country49 |
DISPERSE-2, PLATO, PRASFIT-ACS, TRILOGY ACS and TRITON-TIMI 38 |
43,990 | 29.6 | ACS | DAPT with potent P2Y12 inhibitor vs. clopidogrel after PCI |
Major bleeding (TIMI criteria 1, BARC 2, 3, and 5 or GUSTO bleeding criteria 1) and minor bleeding (TIMI criteria 2) with a median follow-up time of 1.06 years |
No | |
| Spirito et al (2021) Switzerland42 |
Bern PCI Registry | 16,821 | 26 | ACS+CCS | Assessment of the performance of ARC-HBR criteria separately in women and men |
1. Composite of BARC 3 or 5, further stratified into non- access-site and access-site related bleeding at 1 year 2. BARC 2, 3 or 5 bleeding, TIMI and GUSTO bleeding |
No (overall) Nearly Yes (access site by TFI; HR 1.99 [0.96–4.11] P=0.063) No (access site by TRI) |
|
| Yu et al (2016)†3 USA and Europe |
PARIS | 5,018 | 25.5 | ACS+CCS | Investigation of the patterns and impact of DAPT cessation in women and men |
BARC >3 within 2 years | Yes HR 1.39 [1.02–1.89] P=0.04* *Hb value and renal function data were missing and not adjusted |
|
| Vogel et al (2021) Multi-country54 |
TWILIGHT | 9,006 | 23.9 | ACS+CCS | Ticagrelor with vs. without aspirin from the 3rd month after PCI |
Primary; BARC 2, 3, or 5 bleeding at 1 year Secondary; BARC 3 or 5 bleeding, TIMI major bleeding, GUSTO moderate, severe, or life-threatening bleeding or major bleeding as defined by ISTH at 1 year |
No | |
| Xanthopoulou et al (2017)†4 Greece |
GRAPE Registry | 2,047 | 17.6 | ACS | 1-year DAPT after PCI | Every type of BARC bleeding at 1 year |
No (BARC 2–5) Yes (BARC 1; HR 1.58 [1.27–1.96]) |
Data derived from meta-analyses, randomized clinical trials, and registries with focus on sex differences. Studies are presented in alphabetical order of author.†1–4 Citation details are provided in Supplementary Appendix 2. ACS, acute coronary syndrome; ADP, adenine diphosphate; AMI, acute myocardial infarction; ARC-HBR, The Academic Research Consortium for High Bleeding Risk; BARC, Bleeding Academic Research Consortium; BMS, bare metal stent; CABG, coronary artery bypass graft; CAG, coronary angiography; CCS, chronic coronary syndrome; CI, confidence interval; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; GUSTO, Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; Hb, hemoglobin; HR, hazard ratio; IRR, incidence rate ratio; ISTH, International Society of Thrombosis or Hemostasis; LD, loading dose; NR, not recorded; NSTE-ACS, non-ST-elevation-acute coronary syndrome; NSTEMI, non-ST-elevation myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; REPLACE, Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events; STEMI, ST-elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction; TRI, transradial intervention.
DAPT Regimens
The type and duration of DAPT following PCI should be determined by a balance between ischemic and bleeding risks tailored to each patient.
Type of DAPT Regarding the type of DAPT, meta-analyses have demonstrated equivalent safety and efficacy between the sexes43,44 for both clopidogrel and novel P2Y12 inhibitors (ticagrelor, prasugrel, and cangrelor). These findings were observed in trials involving ACS patients.
In the GLOBAL LEADERS trial, female patients with CCS treated with ticagrelor monotherapy showed an elevated bleeding rate up to 1 year compared with the patients under clopidogrel + aspirin 1-year DAPT, and this result suggests cautious use of potent P2Y12 inhibitors such as ticagrelor in female CCS patients.45 However, in Japan, the indication of ticagrelor is limited to ACS, old myocardial infarction and patients who cannot tolerate other P2Y12 inhibitors.
Prasugrel has been approved with a reduced dose (loading and maintenance, 20/3.75 mg) in Japan, because there is an acknowledged bleeding risk among East Asians. The prasugrel post-marketing surveillance in Japan indicated that women did not have a bleeding risk factor from day 31 to 12 months post-PCI, even for CCS patients and the elderly.35 Therefore, sex may not be a significant consideration when using prasugrel, even in CCS patients. Furthermore, a study on the safety and efficacy of a 2.5-mg maintenance dose of prasugrel in Japanese ACS patients at elevated risk of bleeding demonstrated comparable outcomes to the 3.75-mg dose of prasugrel.59 Within that study, women were not identified as independent risk factor for in-hospital major bleeding. Therefore, sex may not be a significant consideration when using prasugrel, possibly with dose reduction criteria, even in CCS patients.
Duration of DAPT Assessing bleeding risk to determine the duration of DAPT, the 2016 ACC/AHA guideline emphasized qualitative bleeding risk factors, and women were identified as a risk factor.2 In contrast, the 2017 ESC guideline and the 2020 JCS Focused Update Guidelines recommend that evaluating high bleeding risk (HBR) should be conducted primarily for the duration of DAPT.1,3 To assess HBR, the 2020 ESC guidelines utilized the PRESICE-DAPT score60 and the ARC-HBR criteria as references.61 The ARC-HBR criteria represent a consensus on a series of clinical and biochemical standards and do not include sex.62 However, it is important to realize that women are more likely to meet the ARC-HBR criteria and consequently have higher ARC-HBR scores than men due to their higher prevalence of factors such as older age, CKD, and anemia.7,42,62 The 2020 JCS Focus Update Guidelines established their own J-HBR criteria. Heart failure, low body weight, peripheral arterial disease, and frailty were included as Japanese-specific factors in addition to the ARC-HBR criteria.3 The J-HBR criteria have been validated as more sensitive but less specific than the original ARC-HBR criteria.63
The 2017 ESC guidelines1 stated that there was no compelling evidence to advocate a regimen for women based on sex-specific differences in both efficacy and safety. In this review, we do not recommend that the type, dosage, and duration of antithrombotic agents should be altered based solely on sex. However, it is crucial to acknowledge that female patients with IHD, particularly Japanese women, often fall into the HBR category due to specific risk factors such as advanced age, low body weight, renal dysfunction, and anemia.3 Therefore, individualized antiplatelet therapies should be considered, tailoring the duration and dosage according to the patient’s specific risk profile.
Anticoagulation Following PCI
Direct Oral Anticoagulants (DOACs) vs. Warfarin Sex differences in the efficacy and safety of DOACs compared with warfarin for patients with atrial fibrillation (AF) have been reported. The 2 meta-analyses of major anticoagulation trials of 4 DOACs64,65 showed no sex-specific differences in the efficacy of stroke prevention between DOACs and warfarin. One of these meta-analyses showed that women treated with DOACs had lower rates of major bleeding compared with men (OR=0.84).64 Similarly, women receiving rivaroxaban in the ROCKET AF trial66 and apixaban in the ARISTOTLE trial67 had a reduced risk of major bleeding complications compared with men after multivariable adjustment (HR=0.82 and 0.74).
Consequently, DOACs may be the preferred anticoagulant for women.
Regarding specific types of DOACs, a meta-analysis of major anticoagulation trials, with warfarin as an indirect comparator, suggested that the safety profile in female patients with AF did not significantly differ for any of the DOACs concerning safety and efficacy.68
Triple Antithrombotic Therapy Approximately 10–15% of patients undergoing PCI for IHD are diagnosed with AF.69 According to the 2020 ESC Guidelines, AF patients with relevant CVD have a CHA2DS2-VASc score of at least 1 (often higher due to the presence of other cardiovascular risk factors) and thus have an indication for DOACs.61,69 Current clinical guidelines recommend triple antithrombotic therapy (TAT), which involves the use of 3 distinct antithrombotic agents for a specified duration as anticoagulation therapy after PCI in patients with AF.1–3 In a recent study, the triple-drug combination therapy (comprising an anticoagulant, clopidogrel, and aspirin) and dual-drug combination therapy (comprising an anticoagulant and clopidogrel) were compared in 69% of patients with AF and undergoing PCI.70 The dual-drug combination therapy treatment significantly reduced the incidence of bleeding complications at 1 year (HR=0.36), and this effect was consistent regardless of sex. On the other hand, an analysis of patients discharged on TAT from the SWEDEHEART registry found that women had a significantly higher rate of early TAT discontinuation due to bleeding compared with men. However, there was no sex difference in the incidence of coronary events, because the study was underpowered to assess potential sex differences in the association between TAT discontinuation and ischemic events due to its relatively small size.71
Study Limitations
Our review has several limitations. We had an emphasis on investigating bleeding complications in women under antithrombotic therapy, rather than post-PCI bleeding complications. Moreover, most analyses focusing on sex differences in RCTs involving DAPT and TAT after PCI have not been conducted to influence treatment strategies, potentially limiting the statistical power of RCT data and leading to false-negative findings. In addition, we presented sex-specific differences in bleeding complications after PCI under antithrombotic therapy using results mostly derived from sub-group and post-hoc analyses of RCTs, which might yield false-positive results compared with prespecified analyses.72 Furthermore, the low representation of women in CVD trials limits the ability to derive sex-specific recommendations. A deeper comprehension of sex-specific variations in clinical outcomes related to antithrombotic therapy post-PCI is essential for developing sex-specific treatment approaches. Future clinical trials should actively incorporate a substantial number of female participants, especially in bleeding-prone populations such as Asians, aiming to establish robust, evidence-based recommendations in this field.
Conclusions
Women appear to have heightened baseline platelet reactivity compared with men, but the clinical significance of this discrepancy on the selection and dosage of antiplatelet agents remains uncertain. Notably, women face an elevated risk of bleeding in the acute phase post-PCI under antiplatelet therapy, but there is no apparent sex disparity in the chronic-phase bleeding risk. To optimize the efficacy of antiplatelet agent and minimize bleeding complications in women, special attention to factors such as age, renal function, weight, and dosing strategy is necessary. Although DOACs may have a lower bleeding risk in women compared with warfarin, there is a lack of evidence to support recommendations for sex differences in TAT regimens.
Our review has identified recent evidence highlighting sex differences in platelet responsiveness and bleeding complications in antithrombotic therapy after PCI. However, recent findings on sex differences in post-PCI bleeding complications did not provide enough evidence to recommend specific therapies for women. Further studies will be needed to formulate sex-sensitive recommendations for post-PCI antithrombotic therapy in future guidelines.
Disclosure
Y.M.N. reports a study grant from Bayer, outside of this study. All other authors have nothing to disclose.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ Contributions
Y.N. screened the records, extracted the data and wrote the manuscript draft. S.T. and Y.M.N. supervised and edited the manuscript. All authors reviewed the final manuscript and approved its contents.
Supplementary Files
Supplementary File Supplementary Appendix 1. Search strategy Supplementary Table. Selection Criteria Supplementary Appendix 2. Additional references
Acknowledgment
The authors express their sincere appreciation to the members of the JCS Joint Working Group for the Guideline on Cardiovascular Practice, With Consideration for Diversity, Equity, and Inclusion, for their significant contributions to the literature searches conducted in this study.
References
- 1. Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Kastrati A, et al.. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur Heart J 2018; 39: 213–260, doi:10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 2. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al.. 2016 ACC/AHA Guideline Focused Update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol 2016; 68: 1082–1115, doi:10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- 3. Nakamura M, Kimura K, Kimura T, Ishihara M, Otsuka F, Kozuma K, et al.. JCS 2020 Guideline focused update on antithrombotic therapy in patients with coronary artery disease. Circ J 2020; 84: 831–865, doi:10.1253/circj.CJ-19-1109. [DOI] [PubMed] [Google Scholar]
- 4. Melloni C, Berger JS, Wang TY, Gunes F, Stebbins A, Pieper KS, et al.. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes 2010; 3: 135–142, doi:10.1161/CIRCOUTCOMES.110.868307. [DOI] [PubMed] [Google Scholar]
- 5. Ding EL.. Sex differences in perceived risks, distrust, and willingness to participate in clinical trials: A randomized study of cardiovascular prevention trials. Arch Intern Med 2007; 167: 905, doi:10.1001/archinte.167.9.905. [DOI] [PubMed] [Google Scholar]
- 6. Jin X, Chandramouli C, Allocco B, Gong E, Lam CSP, Yan LL.. Women’s participation in cardiovascular clinical trials from 2010 to 2017. Circulation 2020; 141: 540–548, doi:10.1161/CIRCULATIONAHA.119.043594. [DOI] [PubMed] [Google Scholar]
- 7. Mehran R, Chandrasekhar J, Urban P, Lang IM, Windhoevel U, Spaulding C, et al.. Sex-based outcomes in patients with a high bleeding risk after percutaneous coronary intervention and 1-month dual antiplatelet therapy: A secondary analysis of the LEADERS FREE randomized clinical trial. JAMA Cardiol 2020; 5: 939, doi:10.1001/jamacardio.2020.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kodaira M, Sawano M, Tanaka M, Kuno T, Numasawa Y, Ueda I, et al.. Female sex as an independent predictor of high bleeding risk among East Asian percutaneous coronary intervention patients: A sex difference analysis. J Cardiol 2021; 78: 431–438, doi:10.1016/j.jjcc.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 9. Saia F, Marino M, Campo G, Valgimigli M, Guastaroba P, Taglieri N, et al.. Incidence and outcome of high on-treatment platelet reactivity in patients with non-ST elevation acute coronary syndromes undergoing percutaneous coronary intervention (from the VIP [VerifyNow and Inhibition of Platelet Reactivity] Study). Am J Cardiol 2013; 112: 792–798, doi:10.1016/j.amjcard.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 10. Gross L, Kupka D, Trenk D, Geisler T, Hadamitzky M, Löw A, et al.. Gender and outcomes following guided de-escalation of antiplatelet treatment in acute coronary syndrome patients: The TROPICAL-ACS Gender Substudy. Thromb Haemost 2019; 119: 1527–1538, doi:10.1055/s-0039-1692441. [DOI] [PubMed] [Google Scholar]
- 11. Ranucci M, Aloisio T, Di Dedda U, Menicanti L, De Vincentiis C, Baryshnikova E, et al.. Gender-based differences in platelet function and platelet reactivity to P2Y12 inhibitors. PLOS ONE 2019; 14: e0225771, doi:10.1371/journal.pone.0225771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu J, Mehran R, Baber U, Ooi SY, Witzenbichler B, Weisz G, et al.. Sex differences in the clinical impact of high platelet reactivity after percutaneous coronary intervention with drug-eluting stents: Results from the ADAPT-DES study (Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents). Circ Cardiovasc Interv 2017; 10: e003577, doi:10.1161/CIRCINTERVENTIONS.116.003577. [DOI] [PubMed] [Google Scholar]
- 13. Clemmensen P, Roe MT, Hochman JS, Cyr DD, Neely ML, McGuire DK, et al.. Long-term outcomes for women versus men with unstable angina/non-ST-segment elevation myocardial infarction managed medically without revascularization: Insights from the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes trial. Am Heart J 2015; 170: 695–705.e5, doi:10.1016/j.ahj.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 14. Cuisset T, Grosdidier C, Loundou AD, Quilici J, Loosveld M, Camoin L, et al.. Clinical implications of very low on-treatment platelet reactivity in patients treated with thienopyridine. JACC Cardiovasc Interv 2013; 6: 854–863, doi:10.1016/j.jcin.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 15. Park DW, Ahn JM, Song HG, Lee JY, Kim WJ, Kang SJ, et al.. Differential prognostic impact of high on-treatment platelet reactivity among patients with acute coronary syndromes versus stable coronary artery disease undergoing percutaneous coronary intervention. Am Heart J 2013; 165: 34–42.e1, doi:10.1016/j.ahj.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 16. Patti G, De Caterina R, Abbate R, Andreotti F, Biasucci LM, Calabrò P, et al.. Platelet function and long-term antiplatelet therapy in women: Is there a gender-specificity? Eur Heart J 2014; 35: 2213–2223, doi:10.1093/eurheartj/ehu279. [DOI] [PubMed] [Google Scholar]
- 17. Wang TY, Angiolillo DJ, Cushman M, Sabatine MS, Bray PF, Smyth SS, et al.. Platelet biology and response to antiplatelet therapy in women. J Am Coll Cardiol 2012; 59: 891–900, doi:10.1016/j.jacc.2011.09.075. [DOI] [PubMed] [Google Scholar]
- 18. Jastrzebska M, Marcinowska Z, Oledzki S, Chelstowski K, Siennicka A, Klysz M, et al.. Variable gender-dependent platelet responses to combined antiplatelet therapy, in patients with stable coronary-artery disease. J Physiol Pharmacol 2018; 69: 595–605, doi:10.26402/jpp.2018.4.10. [DOI] [PubMed] [Google Scholar]
- 19. Holm A, Swahn E, Lawesson SS, Gustafsson KM, Janzon M, Jonasson L, et al.. Sex differences in platelet reactivity in patients with myocardial infarction treated with triple antiplatelet therapy: Results from assessing platelet activity in coronary heart disease (APACHE). Platelets 2021; 32: 524–532, doi:10.1080/09537104.2020.1771550. [DOI] [PubMed] [Google Scholar]
- 20. Nishikawa M, Takeda Y, Isomura N, Tanigawa T, Nanasato M, Tsukahara K, et al.. Association between high platelet reactivity following dual antiplatelet therapy and ischemic events in Japanese patients with coronary artery disease undergoing stent implantation. J Atheroscler Thromb 2020; 27: 13–24, doi:10.5551/jat.48934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ, Metzger DC, et al.. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): A prospective multicentre registry study. Lancet 2013; 382: 614–623, doi:10.1016/S0140-6736(13)61170-8. [DOI] [PubMed] [Google Scholar]
- 22. Aradi D, Kirtane A, Bonello L, Gurbel PA, Tantry US, Huber K, et al.. Bleeding and stent thrombosis on P2Y12-inhibitors: Collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J 2015; 36: 1762–1771, doi:10.1093/eurheartj/ehv104. [DOI] [PubMed] [Google Scholar]
- 23. Breet NJ, Sluman MA, Van Berkel MAJPJ, Van Werkum JW, Bouman HJ, Harmsze AM, et al.. Effect of gender difference on platelet reactivity. Neth Heart J 2011; 19: 451–457, doi:10.1007/s12471-011-0189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verdoia M, Pergolini P, Rolla R, Nardin M, Barbieri L, Daffara V, et al.. Gender differences in platelet reactivity in patients receiving dual antiplatelet therapy. Cardiovasc Drugs Ther 2016; 30: 143–150, doi:10.1007/s10557-016-6646-5. [DOI] [PubMed] [Google Scholar]
- 25. Alexopoulos D, Xanthopoulou I, Storey RF, Bliden KP, Tantry US, Angiolillo DJ, et al.. Platelet reactivity during ticagrelor maintenance therapy: A patient-level data meta-analysis. Am Heart J 2014; 168: 530–536, doi:10.1016/j.ahj.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 26. Zaccardi F, Pitocco D, Willeit P, Laukkanen JA.. Efficacy and safety of P2Y12 inhibitors according to diabetes, age, gender, body mass index and body weight: Systematic review and meta-analyses of randomized clinical trials. Atherosclerosis 2015; 240: 439–445, doi:10.1016/j.atherosclerosis.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 27. Brar SS, Ten Berg J, Marcucci R, Price MJ, Valgimigli M, Kim HS, et al.. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. J Am Coll Cardiol 2011; 58: 1945–1954, doi:10.1016/j.jacc.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 28. Widimsky P, Motovska Z, Bolognese L, Dudek D, Hamm C, Tanguay JF, et al.. Predictors of bleeding in patients with acute coronary syndromes treated with prasugrel. Heart 2015; 101: 1219–1224, doi:10.1136/heartjnl-2015-307686. [DOI] [PubMed] [Google Scholar]
- 29. Hochholzer W, Wiviott SD, Antman EM, Contant CF, Guo J, Giugliano RP, et al.. Predictors of bleeding and time dependence of association of bleeding with mortality: Insights from the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel: Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38). Circulation 2011; 123: 2681–2689, doi:10.1161/CIRCULATIONAHA.110.002683. [DOI] [PubMed] [Google Scholar]
- 30. Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, et al.. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol 2010; 55: 2556–2566, doi:10.1016/j.jacc.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 31. Shah T, Haimi I, Yang Y, Gaston S, Taoutel R, Mehta S, et al.. Meta-analysis of gender disparities in in-hospital care and outcomes in patients with ST-segment elevation myocardial infarction. Am J Cardiol 2021; 147: 23–32, doi:10.1016/j.amjcard.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 32. Simonsson M, Winell H, Olsson H, Szummer K, Alfredsson J, Hall M, et al.. Development and validation of a novel risk score for in-hospital major bleeding in acute myocardial infarction: The SWEDEHEART score. J Am Heart Assoc 2019; 8: e012157, doi:10.1161/JAHA.119.012157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saito S, Isshiki T, Kimura T, Ogawa H, Yokoi H, Nishikawa M, et al.. Impact of arterial access route on bleeding complications in Japanese patients undergoing percutaneous coronary intervention: Insight from the PRASFIT Trial. Circ J 2015; 79: 1928–1937, doi:10.1253/circj.CJ-15-0276. [DOI] [PubMed] [Google Scholar]
- 34. Nakamura M, Iizuka T, Sagawa K, Abe K, Chikada S, Arai M.. Prasugrel for Japanese patients with acute coronary syndrome in short-term clinical practice (PRASFIT-Practice I): A postmarketing observational study. Cardiovasc Interv Ther 2018; 33: 135–145, doi:10.1007/s12928-017-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakamura M, Kitazono T, Kozuma K, Sekine T, Nakamura S, Shiosakai K, et al.. Prasugrel for Japanese patients with ischemic heart disease in long-term clinical practice (PRASFIT-Practice II): 1-year follow-up results of a postmarketing observational study. Circ J 2019; 84: 101–108, doi:10.1253/circj.CJ-19-0645. [DOI] [PubMed] [Google Scholar]
- 36. Shoji S, Sawano M, Sandhu AT, Heidenreich PA, Shiraishi Y, Ikemura N, et al.. Ischemic and bleeding events among patients with acute coronary syndrome associated with low-dose prasugrel vs. standard-dose clopidogrel treatment. JAMA Netw Open 2020; 3: e202004, doi:10.1001/jamanetworkopen.2020.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Numasawa Y, Inohara T, Ishii H, Kuno T, Kodaira M, Kohsaka S, et al.. Comparison of outcomes of women versus men with non-ST-elevation acute coronary syndromes undergoing percutaneous coronary intervention (from the Japanese Nationwide Registry). Am J Cardiol 2017; 119: 826–831, doi:10.1016/j.amjcard.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 38. Nakamura M, Kitazono T, Kozuma K, Sekine T, Nakamura S, Shiosakai K, et al.. Prasugrel for Japanese patients with ischemic heart disease in long-term clinical practice (PRASFIT-Practice II): Final 2-year follow-up results of a postmarketing observational study. Circ J 2020; 84: 1981–1989, doi:10.1253/circj.CJ-20-0253. [DOI] [PubMed] [Google Scholar]
- 39. Numasawa Y, Kohsaka S, Miyata H, Noma S, Suzuki M, Ishikawa S, et al.. Gender differences in in-hospital clinical outcomes after percutaneous coronary interventions: An insight from a Japanese multicenter registry. PLoS One 2015; 10: e0116496, doi:10.1371/journal.pone.0116496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Natsuaki M, Morimoto T, Yamaji K, Watanabe H, Yoshikawa Y, Shiomi H, et al.. Prediction of thrombotic and bleeding events after percutaneous coronary intervention: CREDO-Kyoto thrombotic and bleeding risk scores. J Am Heart Assoc 2018; 7: e008708, doi:10.1161/JAHA.118.008708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kwok CS, Kontopantelis E, Kunadian V, Anderson S, Ratib K, Sperrin M, et al.. Effect of access site, gender, and indication on clinical outcomes after percutaneous coronary intervention: Insights from the British Cardiovascular Intervention Society (BCIS). Am Heart J 2015; 170: 164–172.e5, doi:10.1016/j.ahj.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 42. Spirito A, Gragnano F, Corpataux N, Vaisnora L, Galea R, Svab S, et al.. Sex-based differences in bleeding risk after percutaneous coronary intervention and implications for the Academic Research Consortium High Bleeding Risk Criteria. J Am Heart Assoc 2021; 10: e021965, doi:10.1161/JAHA.121.021965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee KK, Welton N, Shah AS, Adamson PD, Dias S, Anand A, et al.. Differences in relative and absolute effectiveness of oral P2Y12 inhibition in men and women: A meta-analysis and modelling study. Heart 2018; 104: 657–664, doi:10.1136/heartjnl-2017-312003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lau ES, Braunwald E, Murphy SA, Wiviott SD, Bonaca MP, Husted S, et al.. Potent P2Y12 inhibitors in men versus women: A Collaborative Meta-Analysis of Randomized Trials. J Am Coll Cardiol 2017; 69: 1549–1559, doi:10.1016/j.jacc.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 45. Chichareon P, Modolo R, Kerkmeijer L, Tomaniak M, Kogame N, Takahashi K, et al.. Association of sex with outcomes in patients undergoing percutaneous coronary intervention: A subgroup analysis of the GLOBAL LEADERS randomized clinical trial. JAMA Cardiol 2020; 5: 21, doi:10.1001/jamacardio.2019.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gargiulo G, Ariotti S, Santucci A, Piccolo R, Baldo A, Franzone A, et al.. Impact of sex on 2-year clinical outcomes in patients treated with 6-month or 24-month dual-antiplatelet therapy duration. JACC Cardiovasc Interv 2016; 9: 1780–1789, doi:10.1016/j.jcin.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 47. Grodecki K, Huczek Z, Scisło P, Kowara M, Raposeiras-Roubín S, D’Ascenzo F, et al.. Gender-related differences in post-discharge bleeding among patients with acute coronary syndrome on dual antiplatelet therapy: A BleeMACS sub-study. Thromb Res 2018; 168: 156–163, doi:10.1016/j.thromres.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 48. Sawaya FJ, Morice MC, Spaziano M, Mehran R, Didier R, Roy A, et al.. Short-versus long-term dual antiplatelet therapy after drug-eluting stent implantation in women versus men: A sex-specific patient-level pooled-analysis of six randomized trials. Catheter Cardiovasc Interv 2017; 89: 178–189, doi:10.1002/ccd.26653. [DOI] [PubMed] [Google Scholar]
- 49. Schreuder MM, Badal R, Boersma E, Kavousi M, Roos-Hesselink J, Versmissen J, et al.. Efficacy and safety of high potent P2Y12 inhibitors prasugrel and ticagrelor in patients with coronary heart disease treated with dual antiplatelet therapy: A sex-specific systematic review and meta-analysis. J Am Heart Assoc 2020; 9: e014457, doi:10.1161/JAHA.119.014457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, et al.. Coronary thrombosis and major bleeding after PCI with drug-eluting stents. J Am Coll Cardiol 2016; 67: 2224–2234, doi:10.1016/j.jacc.2016.02.064. [DOI] [PubMed] [Google Scholar]
- 51. Husted S, James SK, Bach RG, Becker RC, Budaj A, Heras M, et al.. The efficacy of ticagrelor is maintained in women with acute coronary syndromes participating in the prospective, randomized, PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J 2014; 35: 1541–1550, doi:10.1093/eurheartj/ehu075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee M, Kim DW, Park MW, Lee K, Chang K, Chung WS, et al.. Gender differences in clinical outcomes of acute myocardial infarction undergoing percutaneous coronary intervention: Insights from the KAMIR-NIH Registry. J Geriatr Cardiol 2020; 17: 680–693, doi:10.11909/j.issn.1671-5411.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee CW, Ahn JM, Park DW, Kang SJ, Lee SW, Kim YH, et al.. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: A randomized, controlled trial. Circulation 2014; 129: 304–312, doi:10.1161/CIRCULATIONAHA.113.003303. [DOI] [PubMed] [Google Scholar]
- 54. Vogel B, Baber U, Cohen DJ, Sartori S, Sharma SK, Angiolillo DJ, et al.. Sex differences among patients with high risk receiving ticagrelor with or without aspirin after percutaneous coronary intervention: A subgroup analysis of the TWILIGHT randomized clinical trial. JAMA Cardiol 2021; 6: 1032–1041, doi:10.1001/jamacardio.2021.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Généreux P, Giustino G, Witzenbichler B, Weisz G, Stuckey TD, Rinaldi MJ, et al.. Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol 2015; 66: 1036–1045, doi:10.1016/j.jacc.2015.06.1323. [DOI] [PubMed] [Google Scholar]
- 56. Matteau A, Yeh RW, Camenzind E, Steg PG, Wijns W, Mills J, et al.. Balancing long-term risks of ischemic and bleeding complications after percutaneous coronary intervention with drug-eluting stents. Am J Cardiol 2015; 116: 686–693, doi:10.1016/j.amjcard.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chandrasekhar J, Baber U, Sartori S, Faggioni M, Aquino M, Kini A, et al.. Sex-related differences in outcomes among men and women under 55 years of age with acute coronary syndrome undergoing percutaneous coronary intervention: Results from the PROMETHEUS study. Catheter Cardiovasc Interv 2017; 89: 629–637, doi:10.1002/ccd.26606. [DOI] [PubMed] [Google Scholar]
- 58. Kuno T, Miyamoto Y, Sawano M, Kodaira M, Numasawa Y, Ueda I, et al.. Gender differences in long-term outcomes of young patients who underwent percutaneous coronary intervention: Long-term outcome analysis from a multicenter registry in Japan. Am J Cardiol 2023; 206: 151–160, doi:10.1016/j.amjcard.2023.08.106. [DOI] [PubMed] [Google Scholar]
- 59. Ohya M, Shimada T, Osakada K, Kuwayama A, Miura K, Murai R, et al.. In-hospital bleeding and utility of a maintenance dose of prasugrel 2.5 mg in high bleeding risk patients with acute coronary syndrome. Circ J 2018; 82: 1874–1883, doi:10.1253/circj.CJ-18-0114. [DOI] [PubMed] [Google Scholar]
- 60. Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, et al.. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet 2017; 389: 1025–1034, doi:10.1016/S0140-6736(17)30397-5. [DOI] [PubMed] [Google Scholar]
- 61. Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al.. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021; 42: 1289–1367, doi:10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 62. Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, et al.. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: A Consensus Document from the Academic Research Consortium for High Bleeding Risk. Circulation 2019; 140: 240–261, doi:10.1161/CIRCULATIONAHA.119.040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Natsuaki M, Morimoto T, Shiomi H, Ehara N, Taniguchi R, Tamura T, et al.. Application of the modified high bleeding risk criteria for Japanese patients in an all-comers registry of percutaneous coronary intervention: From the CREDO-Kyoto Registry Cohort-3. Circ J 2021; 85: 769–781, doi:10.1253/circj.CJ-20-0836. [DOI] [PubMed] [Google Scholar]
- 64. Pancholy SB, Sharma PS, Pancholy DS, Patel TM, Callans DJ, Marchlinski FE.. Meta-analysis of gender differences in residual stroke risk and major bleeding in patients with nonvalvular atrial fibrillation treated with oral anticoagulants. Am J Cardiol 2014; 113: 485–490, doi:10.1016/j.amjcard.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 65. Zelniker TA, Ruff CT, Antman EM, Giugliano RP.. The efficacy and safety of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and coronary artery disease: A meta-analysis of randomized trials. Eur Heart J Acute Cardiovasc Care 2019; 8: 554–561, doi:10.1177/2048872618796990. [DOI] [PubMed] [Google Scholar]
- 66. Goodman SG, Wojdyla DM, Piccini JP, White HD, Paolini JF, Nessel CC, et al.. Factors associated with major bleeding events. J Am Coll Cardiol 2014; 63: 891–900, doi:10.1016/j.jacc.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hylek EM, Held C, Alexander JH, Lopes RD, De Caterina R, Wojdyla DM, et al.. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin. J Am Coll Cardiol 2014; 63: 2141–2147, doi:10.1016/j.jacc.2014.02.549. [DOI] [PubMed] [Google Scholar]
- 68. Moseley A, Doukky R, Williams KA, Jaffer AK, Volgman AS.. Indirect comparison of novel oral anticoagulants in women with nonvalvular atrial fibrillation. J Womens Health 2017; 26: 214–221, doi:10.1089/jwh.2016.5892. [DOI] [PubMed] [Google Scholar]
- 69. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al.. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021; 42: 373–498, doi:10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 70. Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman J-P, et al.. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013; 381: 1107–1115, doi:10.1016/S0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- 71. Holm A, Henriksson M, Alfredsson J, Janzon M, Johansson T, Swahn E, et al.. Long term risk and costs of bleeding in men and women treated with triple antithrombotic therapy: An observational study. PLoS One 2021; 16: e0248359, doi:10.1371/journal.pone.0248359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ko D, Rahman F, Martins MAP, Hylek EM, Ellinor PT, Schnabel RB, et al.. Atrial fibrillation in women: Treatment. Nat Rev Cardiol 2017; 14: 113–124, doi:10.1038/nrcardio.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File Supplementary Appendix 1. Search strategy Supplementary Table. Selection Criteria Supplementary Appendix 2. Additional references