Abstract
Mice are routinely used in snake venom research but are costly and subject to pain and suffering. The crustacean Artemia salina could be an alternative to mice, but data to support its adoption in snake venom research is limited. The aim of the present study was to evaluate the suitability of A. salina as a surrogate of mice in assessing the toxicity of venoms and the preclinical efficacy of antivenoms. The toxicity of venoms from 22 snakes of medical importance in sub–Saharan Africa was evaluated in mice (intraperitoneally; i.p. and intravenously; i.v.) and in A. salina. Subsequently, the capacity of a commercial antivenom to neutralize the toxicity of these venoms in mice and A. salina was investigated. There was a positive correlation between the i.v. median lethal doses (LD50s) and the i.p. LD50s in mice (r = 0.804; p < 0.0001), a moderate correlation between the i.v. LD50s in mice and the median lethal concentrations (LC50s) in A. salina (r = 0.606; p = 0.003), and a moderate correlation between the i.p. LD50s in mice and the LC50s in A. salina (r = 0.426; p = 0.048). Moreover, there was a strong correlation between the i.p. median effective doses (ED50s) and the i.v. ED50s in mice (r = 0.941, p < 0.0001), between the i.p. ED50s in mice and the ED50s in A. salina (r = 0.818, p < 0.0001), and between the i.v. ED50s in mice and the ED50s in A. salina (r = 0.972, p < 0.0001). These findings present A. salina as a promising candidate for reducing reliance on mice in snake venom research. Future investigations should build upon these findings, addressing potential limitations and expanding the scope of A. salina in venom research and antivenom development.
Keywords: Antivenom, Artemia salina toxicity test, Neutralization of lethality, Preclinical efficacy, Snake venom
Graphical abstract
Highlights
-
•
The correlation between the mouse lethality and the Artemia salina toxicity tests was assessed using African snake venoms.
-
•
A significant correlation was observed between LD50s when using the intravenous and intraperitoneal routes in mice.
-
•
A significant correlation was observed when comparing LD50s in mice and LC50s in A. salina model.
-
•
A highly significant correlation was observed when comparing antivenom efficacy (ED50) in these two models.
1. Introduction
The biomedical sciences, including anatomy and physiology, disease pathogenesis, surgical technique, and pharmaceutical development, have all benefited greatly from modelling in experimental animals (Bernard, 1957; Robinson et al., 2019). However, the pain, anxiety, distress, and long-term suffering that experimental animals endure creates an ethical dilemma between the use of these animals to further biological research and the welfare of the research subjects (Robinson et al., 2019).
The use of the 3Rs principle—replacement, reduction, and refinement—may result in more humane animal research (Russell and Burch, 1959). Nonetheless, there are certain areas of study where applying this idea has been challenging. For instance, mouse models are the main tool used in snake venom research to assess venom toxicity and the preclinical efficacy of antivenoms (World Health Organization, 2017). Since mice are used frequently in snake venom research, the WHO Guidelines for the Production, Control, and Regulation of Snake Venom Immunoglobulins heavily reference the model despite its limitations in antivenom efficacy evaluation (World Health Organization, 2017; Gutiérrez et al., 2021; Silva et al., 2022).
Mice are injected with different doses of venom in the mouse lethality assay, and they are then monitored for 24–48 h (World Health Organization, 2017). The mortality data obtained from these observations is used to determine the median lethal dose, or LD50—the amount of venom that causes 50% of the injected animals to die (World Health Organization, 2017). On the other hand, mice are injected with an incubated mixture of a constant challenge dose of venom (3–6 LD50s) and graded dilutions of antivenom to study the neutralizing capacity of antivenoms (World Health Organization, 2017). The effectiveness of the antivenom in mitigating venom-induced lethality is then assessed over 24- to 48-h and expressed as the median effective dose (ED50), which is the volume of antivenom or venom/antivenom ratio at which 50% of challenged animals survive (World Health Organization, 2017).
A number of improvements have been made to the mouse lethality test, such as the use of analgesics (Chacón et al., 2015; Herrera et al., 2018), reducing the time of the assay (Barber et al., 2014; Durán et al., 2021), and lowering the number of animals needed to produce reliable results (Solano et al., 2010). Additionally, some authors have presented alternative models, including the use of embryonated eggs (Verity et al., 2021), cell-based assays (Lopes-de-Souza et al., 2019), antivenomics (Gutiérrez et al., 2014; Pla et al., 2017), and in vitro methods such as the indirect hemolytic activity assay (Habermann and Hardt, 1972; Gutiérrez et al., 1988; Barbosa et al., 1995) and the enzyme linked immunosorbent assay (Heneine et al., 1998; Liu et al., 2021). Despite their limitations, these studies have demonstrated noteworthy correlations with the mouse lethality assay for specific venom-antivenom combinations.
Okumu and co-workers have recently used the Artemia salina animal model to determine the neutralization capacity of two antivenoms (Okumu et al., 2020). A follow-up study showed that the A. salina model was better at predicting venom-induced dermonecrosis than lethality in mice (Okumu et al., 2021). This test involves exposing hatched larvae of A. salina to graded doses of venom over 24 h and observing the number of dead larvae (Okumu et al., 2020). The mortality data is used to calculate the median lethal concentration (LC50), i.e., the concentration of venom that causes the death of 50% of A. salina larvae. To determine the neutralizing efficacy of antivenom, the larvae are exposed to a constant challenge concentration of venom mixed with different dilutions of antivenom (Okumu et al., 2020). The number of dead larvae after 24 h is used to calculate the ED50.
The A. salina toxicity test has several advantages over the mouse assay, including ease of use, low cost, quick findings, and the ability to examine a large number of samples, in addition to ethical benefits (Freires et al., 2016). It was first presented by Meyer and colleagues (Meyer et al., 1982), and since then it has been extensively used in toxicology (Kerster and Schaeffer, 1983; Sanchez-Fortun and Barahona, 2009; Hamidi et al., 2014; Freires et al., 2023). However, its application in snake venom research is limited (Okumu et al., 2020, 2021). The present study sought to investigate the suitability of A. salina as a surrogate model for mice in evaluating the toxicity of snake venom and the preclinical efficacy of antivenom.
2. Materials and methods
2.1. Ethics
This study was approved by the Institutional Committee for the Care and Use of Laboratory Animals (CICUA) of Universidad de Costa Rica (reference numbers 82-08 and 39-20) and met the International Guiding Principles for Biomedical Research Involving Animals (Bankowski and Howard-Jones, 1985).
2.2. Venom
The batch numbers and geographical origins of venoms used in this study are summarized in Table 1. The collected venoms were lyophilized and stored at −40 °C. Lyophilized venom was weighed and dissolved in 0.12 M NaCl, 0.04 M phosphate buffer, pH 7.2 (PBS) at the time of use.
Table 1.
Details of the venoms used in toxicity evaluation and preclinical antivenom efficacy assessment.
| Genera | Species | Batch numbera | Geographic origin |
|---|---|---|---|
| Bitis | B. arietans | 322.061 | Unspecified |
| B. gabonica | 725.031 | Unspecified | |
| B. nasicornis | 500.102 | Unspecified | |
| B. rhinoceros | 701.070 | Ghana | |
| Echis | E. leucogaster | 623.070 | Mali |
| E. ocellatus | 216.031 | Unspecified | |
| E. pyramidum | 523.070 | Egypt | |
| Naja | N. ashei | 410.191 | Kenya |
| N. katiensis | 705.010 | Burkina Faso | |
| N. mossambica | 627.002 | Tanzania | |
| N. nigricinta | 507.081 | South Africa | |
| N. nigricollis | 616.031 | Unspecified | |
| N. anchietae | 527.002 | Namibia | |
| N. annulifera | 622.040 | Mozambique | |
| N. haje | 222.061 | Unspecified | |
| N. melanoleuca | 516.031 | Unspecified | |
| N. nivea | 524.010 | South Africa | |
| N. senegalensis | 805.101 | Mali | |
| Dendroaspis | D. angusticeps | 305.000 | Tanzania/Mozambique |
| D. jamesonii | 923.011 | Cameroon | |
| D. polylepis | 416.031 | Unspecified | |
| D. viridis | 516.001 | Ghana |
All venoms were obtained from Latoxan (Portes-dès Valence, France) (https://www.latoxan.com/).
2.3. Snake antivenom
EchiTAb-plus-ICP antivenom (batch 6640421PALQ, which has an expiration date of April 2024 and protein content of 7.3 ± 0.2 g/dL, and batch 6771021PALQ, which has an expiration date of October 2024 and protein content of 7.2 ± 0.1 g/dL) were used in this study. This antivenom is a polyspecific formulation of whole immunoglobulin G (IgG) from the plasma of horses immunized with venoms of B. arietans, E. ocellatus, N. nigricollis, and Dendroaspis polylepis, and purified by caprylic acid precipitation (Rojas et al., 1994). It is effective in neutralizing the venoms of several species of Echis spp, Bitis spp, Naja spp and D. polylepis (Gutiérrez et al., 2005; Segura et al., 2010; Petras et al., 2011).
2.4. Determination of the LD50 of the snake venoms in mice
Groups of eight CD-1 mice of both sexes were pretreated with a 50 mg/kg subcutaneous dose of tramadol (Chacón et al., 2015). After 15 min, the mice received different amounts of venom dissolved in PBS via the intravenous (i.v.) or intraperitoneal (i.p.) route. The weight range of mice that received venom intraperitoneally was between 16 and 18 g, while the weight range of mice that received venom intravenously was between 20 and 22 g. The volume of injection was 0.2 mL for the i.v. route and 0.5 mL for the i.p. route. The number of deaths after 24 h (i.v.) or 48 h (i.p.) was recorded. The LD50 and the corresponding 95% Confidence Intervals (95% CI) were calculated using Probit Regression Analysis (Finney, 1971) and expressed as milligrams of venom per kilogram body weight of mouse (mg venom/kg bwt) that killed 50% of the injected mice.
2.5. Determination of the LC50 of the snake venoms in A. salina
The method of Meyer and colleagues was used with slight modifications (Meyer et al., 1982). Briefly, 1.5 mL of PBS containing different amounts of venoms were mixed with ten 48-hr old A. salina larvae suspended in 0.5 mL of sterile sea water having a NaCl concentration of 0.42 M. These mixtures were incubated at room temperature and the larvae were observed after 24 h. The number of dead larvae, i.e., larvae that did not move during 2 min, was recorded and used to calculate the LC50 by Probit regression analysis (Finney, 1971).
2.6. Determination of the capacity of antivenom to neutralize venom-induced lethality in mice
Aliquots containing a constant challenge dose of venom and variable dilutions of antivenom were incubated at 37 °C for 30 min and injected in mice i.p. or i.v. The challenge dose was 3LD50s for venoms of Naja spp and Dendroaspis spp or 5LD50s for venoms of Bitis spp and Echis spp (Gutiérrez et al., 2005; Segura et al., 2010). Control group mice received venom only dissolved in PBS. The volume of injection was 0.2 mL for the i.v. route and 0.5 mL for the i.p. route. The number of deaths were recorded after 24 h (when the i.v. route was used) or 48 h (when the i.p. route was used). The ED50, expressed as mg venom/mL of antivenom, and the corresponding 95% confidence intervals (CI), were calculated by Probit Regression Analysis (Finney, 1971).
2.7. Determination of the capacity of the antivenom to neutralize venom-induced lethality in A. salina
The method described by Okumu et al. was used (Okumu et al., 2020), with modifications. Briefly, 0.5 mL of PBS, containing a constant challenge dose of venoms (2–6 LC50s depending on the venom), were mixed with 1.0 mL of different dilutions of the antivenom. Antivenom was previously dialyzed using a dialysis tubing cellulose membrane (D9527; Sigma Aldrich, St Louis, MO, USA) in a volume of distilled water corresponding to 40 times the volume of antivenom (for the first two cycles), and 0.15 M NaCl (for the last cycle) to remove phenol (a preservative), as it is toxic to the A. salina larvae. The antivenom was then concentrated by freeze-drying and dissolved in distilled water to attain the same protein concentration as the original antivenom. Venom-antivenom mixtures were prepared and incubated at room temperature for 30 min. Then, mixtures were centrifuged at 17,700×g for 6 min to avoid interference in the assay due to turbidity and immune complex precipitation. Ten A. salina larvae were suspended in 0.5 mL of sterile sea water and added to the venom-antivenom mixtures (1.5 mL), followed by incubation for 24 h at room temperature. A. salina larvae exposed to venom only served as control. Mortality was recorded after 24 h and the ED50 and 95% CI were calculated by probit regression analysis. ED50 corresponds to the venom/antivenom ratio at which 50% of the A. salina larvae survived.
2.8. Statistical analysis
The LD50 and LC50 of the venoms and the ED50 of the antivenom (and their corresponding 95% confidence intervals) were determined by Probit Regression Analysis. Pearson's bivariate correlations evaluated the relationship between lethality in mice (i.v./i.p. LD50) and lethality in A. salina (LC50) as well as the relationship between the ED50 of antivenom determined from mice and A. salina. In the case of venoms which were not neutralized by the antivenom, i.e., the values of ED50s could not be calculated, these data were not used for the correlation analysis. Data analysis was carried out using the Statistical Package for the Social Sciences (IBM, Version 25).
3. Results
3.1. Toxicity of the venoms in mice and in A. salina
Table 2 shows the results for the i.v. LD50, i.p. LD50, and LC50 of the 22 studied venoms. According to the i.v. LD50 data, D. polylepis venom was the most toxic with an LD50 of 0.31 mg/kg (0.29–0.35) while N. annulifera venom was the least toxic with an LD50 of 3.47 mg/kg (2.76–5.44). According to the i.p. LD50 data, D. polylepis venom was the most toxic with an LD50 of 0.26 mg/kg (0.18–0.34) while N. anchietae was the least toxic with an LD50 of 3.85 mg/kg (2.84–4.99) (Table 2). The i.p. LD50/i.v. LD50 ratio for viperids ranged from 1.41 (B. gabonica) to 3.70 (E. pyramidum) while in elapids it ranged from 0.80 (N. haje) to 1.74 (N. nigricinta). Moreover, according to LC50 data from the A. salina model, D. angusticeps, D. jamesoni, D. viridis, and N. nigricollis venoms were the most toxic and had similar LC50s, i.e. 0.01 (0.00–0.01) mg/mL for D. angusticeps, 0.01 (0.00–0.02) mg/mL for D. jamesonii, 0.01 (0.00–0.02) mg/mL for D. viridis, and 0.01 (0.00–0.02) mg/mL for N. nigricollis, while N. annulifera venom was the least toxic venom with an LC50 of 0.24 (0.16–0.34) mg/mL (Table 2).
Table 2.
A comparison of the toxicity (lethality) of venom from 22 snakes of medical importance in sub-Saharan Africa.
| Genera | Species | Mouse |
A. salina |
||
|---|---|---|---|---|---|
| i.v. LD50 (mg/kg) | i.p. LD50 (mg/kg) | i.p. LD50/iv. LD50 | LC50 (mg/mL) | ||
| Bitis | B. arietans | 0.54 (0.40–0.75) | 1.23 (0.98–1.49) | 2.28 | 0.05 (0.02–0.07) |
| B. gabonica | 0.98 (0.86–1.12) | 1.38 (0.93–1.85) | 1.41 | 0.07 (0.04–0.10) | |
| B. nasicornis | 0.92 (0.82–1.09) | 1.48 (0.86–1.95) | 1.61 | 0.09 (0.06–0.16) | |
| B. rhinoceros | 0.85 (0.72–0.97) | 1.42 (0.94–2.08) | 1.67 | 0.05 (0.02–0.09) | |
| Echis | E. leucogaster | 1.44 (1.04–1.96) | 2.31 (1.55–3.07) | 1.60 | 0.09 (0.04–0.13) |
| E. ocellatus | 0.87 (0.81–0.94) | 1.84 (1.24–2.89) | 2.11 | 0.05 (0.04–0.07) | |
| E. pyramidum | 0.61 (0.45–0.83) | 2.26 (1.51–3.84) | 3.70 | 0.05 (0.04–0.07) | |
| Naja | N. ashei | 0.88 (0.61–1.24) | 1.26 (0.76–1.98) | 1.43 | 0.02 (0.01–0.04) |
| N. katiensis | 0.88 (0.65–1.18) | 1.23 (0.86–1.79) | 1.40 | 0.02 (0.01–0.03) | |
| N. mossambica | 0.99 (0.87–1.14) | 1.08 (0.81–1.45) | 1.09 | 0.02 (0.01–0.04) | |
| N. nigricinta | 0.77 (0.67–0.88) | 1.34 (0.86–2.24) | 1.74 | 0.04 (0.02–0.06) | |
| N. nigricollis | 0.94 (0.84–1.07) | 1.08 (0.63–1.55) | 1.15 | 0.01 (0.00–0.02) | |
| N. anchietae | 2.46 (1.66–3.72) | 3.85 (2.84–4.99) | 1.57 | 0.04 (0.02–0.06) | |
| N. annulifera | 3.47 (2.76–5.44) | 3.03 (1.77–4.34) | 0.87 | 0.24 (0.16–0.34) | |
| N. haje | 0.56 (0.44–0.85) | 0.45 (0.35–0.49) | 0.80 | 0.05 (0.03–0.08) | |
| N. melanoleuca | 0.33 (0.25–0.44) | 0.48 (0.38–0.69) | 1.45 | 0.07 (0.05–0.10) | |
| N. nivea | 1.59 (1.37–1.84) | 1.32 (1.05–1.66) | 0.83 | 0.02 (0.01–0.04) | |
| N. senegalensis | 0.50 (0.35–0.64) | 0.45 (0.35–0.61) | 0.90 | 0.02 (0.00–0.01) | |
| Dendroaspis | D. angusticeps | 1.40 (1.23–1.60) | 2.10 (1.76–2.31) | 1.50 | 0.01 (0.00–0.01) |
| D. jamesonii | 1.01 (0.93–1.19) | 1.09 (0.84–1.56) | 1.08 | 0.01 (0.01–0.02) | |
| D. polylepis | 0.31 (0.29–0.35) | 0.26 (0.18–0.34) | 0.84 | 0.02 (0.01–0.03) | |
| D. viridis | 0.43 (0.32–0.74) | 0.52 (0.48–0.57) | 1.20 | 0.01 (0.00–0.02) | |
*Lethality was expressed as the Median Lethal Dose (LD50) in the mouse model (mg/kg body weight), or the median Lethal Concentration (LC50) (mg/mL) in the brine shrimp model. In the three cases, the 95% CIs are shown in parenthesis.
3.2. Relationship between the lethality of the venoms in mice and A. salina
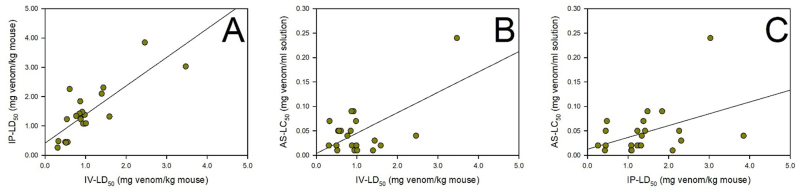
There was a strong and significant correlation between the i.v. LD50 and the i.p. LD50 of the studied venoms in mice (r = 0.804, n = 22, p < 0.0001) (Fig. 1A), and a moderate and significant correlation between the i.v. LD50 values of the venoms in mice and the LC50 of the venoms in A. salina (r = 0.606, n = 22, p = 0.003) (Fig. 1B). There was a moderate and significant correlation between the i.p. LD50 values of the venoms in mice and the LC50 of the venoms in A. salina (r = 0.426, n = 22, p = 0.048) (Fig. 1C).
Fig. 1.
Pearson's bivariate correlations between the i.v. LD50 and i.p. LD50 of snake venoms in mice (r = 0.804, n = 22, p < 0.0001) (A), the iv LD50 in mice and the LC50 in A. salina (r = 0.606, n = 22, p < 0.003) (B), and the i.p. LD50 and the LC50 in A. salina (r = 0.426, n = 22, p < 0.048) (C). i.v.: intravenous, i.p.: intraperitoneal, LD50: median lethal dose, LC50: median lethal concentration.
3.3. Neutralization capacity of antivenom against venom-induced lethality in mice and A. salina
According to neutralization data from the i.v. ED50 protocol, the test antivenom was most effective in neutralizing E. ocellatus, B. arietans, and B. rhinoceros venoms but did not neutralize the venoms of D. angusticeps, D. jamesoni, N. nivea, N. anchieta, N. annulifera, B. nasicornis and E. leucogaster at the lowest venom/antivenom ratios tested (Table 3). According to neutralization data from the i.p. ED50 protocol, the test antivenom was most effective in neutralizing B. arietans, E. ocellatus, and E. pyramidum venoms but did not neutralize the venoms of N. haje, N. anchietae, N. annulifera, D. angusticeps, and D. jamesoni at the lowest venom/antivenom ratios tested (Table 3). According to neutralization data from the ED50 protocol in A. salina, the test antivenom was the most effective in neutralizing E. ocellatus, B. arietans, and B. rhinoceros venoms but failed to neutralize D. angusticeps and D. jamesoni venoms. (Table 3).
Table 3.
Capacity of the antivenom to neutralize venom-induced lethality in mice and A. salina.
| Genera | Species | Animal model |
||
|---|---|---|---|---|
| Mousea |
A. salinab |
|||
| i.v. ED50 | i.p. ED50 | ED50 | ||
| Bitis | B. arietans | 5.00 (4.00–7.20) | 5.50 (4.40–7.00) | 3.30 (2.10–6.70) |
| B. gabonica | 1.20 (0.70–2.30) | 3.00 (1.90–5.00) | 1.50 (0.20–3.70) | |
| B. nasicornis | <0.75 | 3.20 (1.90–5.00) | 0.75 (0.03–1.40) | |
| B. rhinoceros | 3.00 (2.00–5.60) | 3.40 (2.70–4.40) | 3.20 (2.10–5.20) | |
| Echis | E. leucogaster | <3.00 | 3.20 (1.80–5.10) | 1.60 (0.80–4.50) |
| E. ocellatus | 5.10 (3.90–6.90) | 4.30 (2.90–5.70) | 4.10 (2.00–6.60) | |
| E. pyramidum | 2.90 (2.10–3.80) | 3.90 (2.50–4.90) | 2.80 (1.40–5.10) | |
| Naja | N. ashei | 0.49 (0.37–0.59) | 0.89 (0.60–1.31) | 0.90 (0.60–1.50) |
| N. katiensis | 0.73 (0.55–0.99) | 0.36 (0.25–0.53) | 1.10 (0.60–2.00) | |
| N. mossambica | 0.84 (0.62–1.59) | 0.58 (0.33–0.87) | 1.20 (0.80–1.70) | |
| N. nigricinta | 0.69 (0.54–0.89) | 0.67 (0.42–0.94) | 1.00 (0.80–1.40) | |
| N. nigricollis | 1.00 (0.70–1.50) | 0.74 (0.33–1.11) | 1.50 (0.90–2.00) | |
| N. anchietae | <0.60 | <1.00 | 0.80 (0.10–2.00) | |
| N. annulifera | <1.00 | <0.70 | 0.60 (0.10–1.20) | |
| N. haje | 0.10 (0.07–0.20) | <0.08 | 0.90 (0.10–2.50) | |
| N. melanoleuca | 0.16 (0.09–0.25) | 0.17 (0.02–0.38) | 1.00 (0.30–1.80) | |
| N. nivea | <1.00 | 0.47 (0.20–0.81) | 1.20 (0.50–2.20) | |
| N. senegalensis | 0.10 (0.00–0.20) | 0.12 (0.06–0.18) | 0.90 (0.10–2.00) | |
| Dendroaspis | D.angusticeps | <0.80 | <0.40 | <0.19 |
| D. jamesonii | <0.30 | <0.10 | <0.25 | |
| D. polylepis | 0.10 (0.00–0.20) | 0.10 (0.00–0.10) | 0.50 (0.10–1.00) | |
| D. viridis | 0.16 (0.11–0.21) | 0.10 (0.04–0.16) | 0.70 (0.40–1.30) | |
mg venom/ml antivenom.
mg venom/ml antivenom*, ED50: Median Effective Dose.
3.4. Relationship between neutralization of venom-induced lethality by antivenom in mice and A. salina
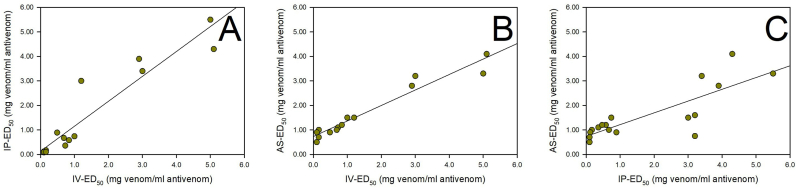
A strong, positive, and significant correlation was observed between the i.p. ED50 and the i.v. ED50 in mice (r = 0.941, n = 14, p < 0.0001) (Fig. 2A), between the i.v. ED50 in mice and the ED50 in A. salina (r = 0.972, n = 15, p < 0.0001) (Fig. 2B), and between the i.p. ED50 in mice and the ED50 in A. salina (r = 0.818, n = 17, p < 0.0001) (Fig. 2C).
Fig. 2.
Pearson's bivariate correlations between the i.v. ED50 and i.p. ED50 in mice (r = 0.941, n = 14, p < 0.0001) (A), the i.v. ED50 in mice and the EC50 in A. salina (r = 0.972, n = 15, p < 0.0001) (B), and the i.p. ED50 in mice and the EC50 in A. salina (r = 0.818, n = 17, p < 0.0001) (C). i.v.: intravenous, i.p.: intraperitoneal, ED50: median effective dose.
4. Discussion
The present study used mice and A. salina to assess the toxicity of 22 venoms from snakes of medical importance in sub–Saharan Africa. The efficacy of an antivenom routinely used in clinical practice in the region was also investigated via the two models, and the results using these models were compared to establish whether they correlated. In terms of lethality in the mouse model, our results allow the comparison between LD50 values by the i.v. and the i.p. routes. As a general trend, viperid venoms of the genera Bitis and Echis tend to be more toxic by the i.v. route as compared to the i.p. route, although it was observed that only in the cases of B. arietans, E. ocellatus and E. pyramidum was this difference significant, i.e., the 95% CI did not overlap. In the case of elapid venoms of the genera Naja and Dendroaspis, the differences between values of LD50 by these routes were less marked, and only in the case of D. angusticeps venom was there a significant difference, i.e., a lower value by the i.p. route was observed. The general trend observed towards less marked differences between lethality by these two routes of venom administration in the case of elapid venoms can be explained by the fact that lethal toxins in elapids are low molecular weight neurotoxins (6–9 kDa) known to have higher bioavailability regardless of the route of injection (Oukkache et al., 2014).
Correlations were observed between the toxicity of the venoms in mice and in A. salina. The mechanism of venom-induced lethality in A. salina is not clear. However, in the case of predominantly neurotoxic elapids, venom-induced lethality may be due to three finger neurotoxins acting at the neuromuscular junctions. On the other hand, the cytotoxic activities of snake venom metalloproteases (SVMPs), phospholipase A2s (PLA2s) and cytotoxic three finger toxins (3FTxs) in predominantly cytotoxic elapids and viperids may be responsible for venom-induced lethality probably through the damage of tissues in A. salina. Further work is necessary to identify the components in a variety of venoms which are responsible for toxicity in A. salina, in order to have an in-depth characterization of this experimental model of toxicity. This could be achieved by determining the ‘toxicity score’ of venom fractions in A. salina (Laustsen et al., 2015).
Viperid venom-induced lethality in mice after i.v. administration has been associated with the procoagulant effects of the venom, which causes rapid intravascular thrombosis induced by the procoagulant snake venom serine proteases (SVSPs) and metalloproteases (SVMPs) (Gutiérrez et al., 2017; Offor et al., 2022). In addition, massive systemic hemorrhage induced by SVMPs and toxins affecting hemostasis may also contribute to lethality by the i.v. route. On the other hand, viper venom-induced lethality in mice after i.p. administration is likely a consequence of massive extravasation secondary to the hemorrhagic action of SVMPs and the increase in vascular permeability induced by SVMPs, SVSPs and PLA2s (Chacón et al., 2015; Gutiérrez et al., 2017). However, other mechanisms might play a role in lethality, and these may vary from venom to venom. It is not clear how these different mechanisms converge resulting in the significant correlations observed in LD50 when the i.v. or i.p. routes are used.
The most striking finding of our study was the high correlation between mouse and A. salina models when the neutralizing ability of the antivenom was evaluated. It is noteworthy that the correlations between the models were higher when comparing the values of ED50s than when comparing the values of LD50s. Therefore, regardless of the mechanisms of toxicity operating in the two models, the high correlation described for the estimation of ED50 values of the antivenom suggests that the A. salina toxicity assay could be a suitable alternative to the mouse assays in some stages of the routine quality control of antivenoms, for example, in the assessment of the neutralizing ability of raw hyperimmune plasma and the verification of the specification fulfillment of bulk batches. In the context of the 3Rs principle, the A. salina toxicity assay may represent a positive step forward in the road to replace the mouse lethality assays in the assessment of venom toxicity and antivenom efficacy.
Since the phenol present in antivenoms is toxic to A. salina, we introduced a dialysis step to remove this preservative. Then, the antivenom was concentrated (in our case by freeze-drying followed by resuspension in water) to attain the same protein concentration as the original antivenom. We recommend using this, or a similar, protocol when testing phenol-containing antivenoms for their neutralizing efficacy using the A. salina model. This is not necessary when tests are done in mice because phenol, at the concentration used in antivenom, is not toxic to mice.
In conclusion, our observations strongly suggest that the A. salina model is a promising candidate for reducing reliance on mice in snake venom research and antivenom quality control. Future investigations should build upon these findings, addressing potential limitations and expanding the scope of A. salina in venom research and antivenom development.
Ethical statement
This study was approved by the Institutional Committee for the Care and Use of Laboratory Animals (CICUA) of Universidad de Costa Rica (reference numbers 82-08 and 39-20).
CRediT authorship contribution statement
Xavier Araya: Writing – review & editing, Writing – original draft, Methodology, Investigation, Conceptualization. Mitchel Okumu: Writing – review & editing, Writing – original draft, Methodology. Gina Durán: Writing – review & editing, Methodology, Investigation. Aarón Gómez: Writing – review & editing, Writing – original draft, Methodology, Investigation. José María Gutiérrez: Writing – review & editing, Writing – original draft, Project administration, Funding acquisition. Guillermo León: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Gina Durán, Aarón Gómez, José María Gutiérrez and Guillermo León work at Instituto Clodomiro Picado, University of Costa Rica, where the antivenom used in this study is manufactured.
Acknowledgements
This study was supported by Wellcome Trust [Reference 220517/Z/20/Z] and Vicerrectoría de Investigación, Universidad de Costa Rica [projects 741-A0-804 and 741-C0-523]. The authors thank our colleagues at Instituto Clodomiro Picado for their collaboration in this study.
Handling Editor: Ray Norton
Data availability
Data will be made available on request.
References
- Bankowski Z., Howard-Jones N. CIOMS (Council of International Organizations of medical sciences) International guiding principles for biomedical research involving animals. Geneva. 1985 [Google Scholar]
- Barber C.M., Madaras F., Turnbull R.K., Morley T., Dunstan N., Allen L., Kuchel T., Mirtschin P., Hodgson W.C. Comparative studies of the venom of a new Taipan species, Oxyuranus temporalis, with other members of its genus. Toxins. 2014;6(7):1979–1995. doi: 10.3390/toxins6071979. PMID: 24992081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa C.F., Rodrigues R.J., Olortegui C.C., Sanchez E.F., Heneine L.G. Determination of the neutralizing potency of horse antivenom against bothropic and crotalic venoms by indirect enzyme immunoassay. Braz. J. Med. Biol. Res. 1995;28(10):1077–1080. PMID: 8634680. [PubMed] [Google Scholar]
- Bernard C. Dover Publications; New York: 1957. An Introduction to the Study of Experimental Medicine; p. 226. [Google Scholar]
- Chacón F., Oviedo A., Escalante T., Solano G., Rucavado A., Gutiérrez J.M. The lethality test used for estimating the potency of antivenoms against Bothrops asper snake venom: pathophysiological mechanisms, prophylactic analgesia, and a surrogate in vitro assay. Toxicon. 2015;93:41–50. doi: 10.1016/j.toxicon.2014.11.223. PMID: 25447772. [DOI] [PubMed] [Google Scholar]
- Durán G., Solano G., Gómez A., Cordero D., Sánchez A., Villalta M., Sánchez M., Díaz C., Gutiérrez J.M., León G. Assessing a 6-h endpoint observation time in the lethality neutralization assay used to evaluate the preclinical efficacy of snake antivenoms. Toxicon. 2021;X 12 doi: 10.1016/j.toxcx.2021.100087. PMID: 34888521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney D.J. Cambridge University Press; Cambridge, UK: 1971. Probit Analysis. [Google Scholar]
- Freires I.A., Morelo D.F.C., Soares L.F.F., Costa I.S., de Araújo L.P., Breseghello I., Abdalla H.B., Lazarini J.G., Rosalen P.L., Pigossi S.C., Franchin M. Progress and promise of alternative animal and non-animal methods in biomedical research. Arch. Toxicol. 2023;97(9):2329–2342. doi: 10.1007/s00204-023-03532-1. PMID: 37394624. [DOI] [PubMed] [Google Scholar]
- Freires I.A., Sardi J.C., de Castro R.D., Rosalen P.L. Alternative animal and non-animal models for drug discovery and development: Bonus or burden? Pharm. Res. (N. Y.) 2016;34(4):681–686. doi: 10.1007/s11095-016-2069-z. PMID: 27858217. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., Avila C., Rojas E., Cerdas L. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon. 1988;26(4):411–413. doi: 10.1016/0041-0101(88)90010-4. PMID: 3406951. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., Lomonte B., Sanz L., Calvete J.J., Pla D. Immunological profile of antivenoms: preclinical analysis of the efficacy of a polyspecific antivenom through antivenomics and neutralization assays. J Proteomics. 2014;105:340–350. doi: 10.1016/j.jprot.2014.02.021. PMID: 24583507. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., Rojas E., Quesada L., León G., Núñez J., Laing G.D., Sasa M., Renjifo J.M., Nasidi A., Warrell D.A., Theakston R.D., Rojas G. Pan-African polyspecific antivenom produced by caprylic acid purification of horse IgG: an alternative to the antivenom crisis in Africa. Trans. R. Soc. Trop. Med. Hyg. 2005;99(6):468–475. doi: 10.1016/j.trstmh.2004.09.014. PMID: 15837359. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., Solano G., Pla D., Herrera M., Segura Á., Vargas M., Villalta M., Sánchez A., Sanz L., Lomonte B., León G., Calvete J.J. Preclinical evaluation of the efficacy of antivenoms for snakebite envenoming: state-of-the-art and challenges ahead. Toxins. 2017;9(5):163. doi: 10.3390/toxins9050163. PMID: 28505100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J.M., Vargas M., Segura Á., Herrera M., Villalta M., Solano G., Sánchez A., Herrera C., León G. In vitro tests for assessing the neutralizing ability of snake antivenoms: toward the 3Rs principles. Front. Immunol. 2021;11 doi: 10.3389/fimmu.2020.617429. PMID: 33505403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann E., Hardt K.L. A sensitive and specific plate test for the quantitation of phospholipases. Anal. Biochem. 1972;50(1):163–173. doi: 10.1016/0003-2697(72)90495-2. PMID: 4342994. [DOI] [PubMed] [Google Scholar]
- Hamidi M., Jovanova B., Kadifkova Panovska T. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014;60:9–18. doi: 10.33320/mace.pharm.bull.2014.60.01.002. [DOI] [Google Scholar]
- Heneine L.G., Carvalho A.D., Jr., Barbosa C.F., Arávjo dos Santos M.R. Development of an ELISA to assess the potency of horse therapeutic polyvalent antibothropic antivenom. Toxicon. 1998;36(10):1363–1370. doi: 10.1016/s0041-0101(98)00014-2. PMID: 9723835. [DOI] [PubMed] [Google Scholar]
- Herrera C., Bolton F., Arias A.S., Harrison R.A., Gutiérrez J.M. Analgesic effect of morphine and tramadol in standard toxicity assays in mice injected with venom of the snake Bothrops asper. Toxicon. 2018;154:35–41. doi: 10.1016/j.toxicon.2018.09.012. PMID: 30268394. [DOI] [PubMed] [Google Scholar]
- Kerster H.W., Schaeffer D.J. Brine shrimp (Artemia salina) nauplii as a teratogen test system. Ecotoxicol. Environ. Saf. 1983;7(3):342–349. doi: 10.1016/0147-6513(83)90079-9. PMID: 6872918. [DOI] [PubMed] [Google Scholar]
- Laustsen A.H., Lohse B., Lomonte B., Engmark M., Gutiérrez J.M. Selecting key toxins for focused development of elapid snake antivenoms and inhibitors guided by a Toxicity Score. Toxicon. 2015;104:43–45. doi: 10.1016/j.toxicon.2015.07.334. Epub 2015 Jul 31. PMID: 26238171. [DOI] [PubMed] [Google Scholar]
- Liu C.C., Hsiao Y.C., Chu L.J., Wang P.J., Liu C.H., Hsieh W.C., Yu J.S. Development of antibody detection ELISA based on immunoreactive toxins and toxin-derived peptides to evaluate the neutralization potency of equine plasma against Naja atra in Taiwan. Toxins. 2021;13(11):818. doi: 10.3390/toxins13110818. PMID: 34822602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-de-Souza L., Costal-Oliveira F., Stransky S., Fonseca de Freitas C., Guerra-Duarte C., Braga V.M.M., Chávez-Olórtegui C. Development of a cell-based in vitro assay as a possible alternative for determining bothropic antivenom potency. Toxicon. 2019;170:68–76. doi: 10.1016/j.toxicon.2019.09.010. PMID: 31494208. [DOI] [PubMed] [Google Scholar]
- Meyer B.N., Ferrigni N.R., Putnam J.E., Jacobsen L.B., Nichols D.E., McLaughlin J.L. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 1982;45(5):31–34. doi: 10.1055/s-2007-971236. PMID: 17396775. [DOI] [PubMed] [Google Scholar]
- Offor B.C., Muller B., Piater L.A. A review of the proteomic profiling of African viperidae and elapidae snake venoms and their antivenom neutralisation. Toxins. 2022;14(11):723. doi: 10.3390/toxins14110723. PMID: 36355973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumu M.O., Mbaria J.M., Gikunju J.K., Mbuthia P.G., Madadi V.O., Ochola F.O. Enzymatic activity and brine shrimp lethality of venom from the large brown spitting cobra (Naja ashei) and its neutralization by antivenom. BMC Res. Notes. 2020;13(1):325. doi: 10.1186/s13104-020-05167-2. PMID: 32631407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumu M.O., Mbaria J.M., Gikunju J.K., Mbuthia P.G., Madadi V.O., Ochola F.O., Jepkorir M.S. Artemia salina as an animal model for the preliminary evaluation of snake venom-induced toxicity. Toxicon. 2021;X 12 doi: 10.1016/j.toxcx.2021.100082. PMID: 34471870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oukkache N., El Jaoudi R., Ghalim N., Chgoury F., Bouhaouala B., Mdaghri N.E., Sabatier J.M. Evaluation of the lethal potency of scorpion and snake venoms and comparison between intraperitoneal and intravenous injection routes. Toxins. 2014;6(6):1873–1881. doi: 10.3390/toxins6061873. PMID: 24926799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petras D., Sanz L., Segura A., Herrera M., Villalta M., Solano D., Vargas M., León G., Warrell D.A., Theakston R.D., Harrison R.A., Durfa N., Nasidi A., Gutiérrez J.M., Calvete J.J. Snake venomics of African spitting cobras: toxin composition and assessment of congeneric cross-reactivity of the pan-African EchiTAb-Plus-ICP antivenom by antivenomics and neutralization approaches. J. Proteome Res. 2011;10(3):1266–1280. doi: 10.1021/pr101040f. PMID: 21171584. [DOI] [PubMed] [Google Scholar]
- Pla D., Rodríguez Y., Calvete J.J. Third generation antivenomics: Pushing the limits of the in vitro preclinical assessment of antivenoms. Toxins. 2017;9(5):158. doi: 10.3390/toxins9050158. PMID: 28489039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N.B., Krieger K., Khan F.M., Huffman W., Chang M., Naik A., Yongle R., Hameed I., Krieger K., Girardi L.N., Gaudino M. The current state of animal models in research: a review. Int. J. Surg. 2019;72:9–13. doi: 10.1016/j.ijsu.2019.10.015. PMID: 31627013. [DOI] [PubMed] [Google Scholar]
- Rojas G., Jiménez J.M., Gutiérrez J.M. Caprylic acid fractionation of hyperimmune horse plasma: description of a simple procedure for antivenom production. Toxicon. 1994;32(3):351–363. doi: 10.1016/0041-0101(94)90087-6. PMID: 8016856. [DOI] [PubMed] [Google Scholar]
- Russell W.M.S., Burch R.L. Methuen; 1959. The Principles of Humane Experimental Technique. [Google Scholar]
- Sánchez-Fortún S., Barahona M.V. Toxicity and characterization of cholinesterase-inhibition induced by diisopropyl fluorophosphate in Artemia salina larvae. Ecotoxicol. Environ. Saf. 2009;72(3):775–780. doi: 10.1016/j.ecoenv.2007.11.004. PMID: 18191451. [DOI] [PubMed] [Google Scholar]
- Segura A., Villalta M., Herrera M., León G., Harrison R., Durfa N., Nasidi A., Calvete J.J., Theakston R.D., Warrell D.A., Gutiérrez J.M. Preclinical assessment of the efficacy of a new antivenom (EchiTAb-Plus-ICP) for the treatment of viper envenoming in sub-Saharan Africa. Toxicon. 2010;55(2–3):369–374. doi: 10.1016/j.toxicon.2009.08.010. PMID: 19699756. [DOI] [PubMed] [Google Scholar]
- Silva A., Hodgson W.C., Tasoulis T., Isbister G.K. Rodent lethality models are problematic for evaluating antivenoms for human envenoming. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.830384. PMID: 35185582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano G., Segura A., Herrera M., Gómez A., Villalta M., Gutiérrez J.M., León G. Study of the design and analytical properties of the lethality neutralization assay used to estimate antivenom potency against Bothrops asper snake venom. Biologicals. 2010;38(5):577–585. doi: 10.1016/j.biologicals.2010.05.006. PMID: 20638298. [DOI] [PubMed] [Google Scholar]
- Verity E.E., Stewart K., Vandenberg K., Ong C., Rockman S. Potency testing of venoms and antivenoms in embryonated eggs: an ethical alternative to animal testing. Toxins. 2021;13(4):233. doi: 10.3390/toxins13040233. PMID: 33805138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2017. WHO Guidelines for the Production Control and Regulation of Snake Antivenom Immunoglobulins. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.