Abstract
Purpose
To report a 15 year old girl with citrullinemia type 1 and 2 accompanied by neurologic signs and symptoms and a novel ocular complaint in cornea like tyrosinemia type 2.
Observations
A 15 year old female was admitted with decreased consciousness and neurologic signs and symptoms. Citrulinemia was discovered through metabolic testing. Later genetic studies revealed mutations in both ASS1 and SLC25A13 genes. Two years after the first presentation, the patient was re-admitted with complaints of bilateral photophobia and tearing. Biomicroscopic examination revealed bilateral corneal haziness with pseudodendritic lesions like tyrosinemia type 2 that were subsided with protein restriction and the use of urea cycle disease (UCD) formula.
Conclusions and importance
Citrullinemia is the inherited autosomal recessive disorder of urea cycle that leads to ammonia and accumulation of other toxic substances in the blood. Two types of Citrullinemia have been defined. Citrullinemia type 1, caused by deficiency or reduction in argininosuccinate synthetase enzyme activity due to damaging mutation in ASS1 gene. Citrullinemia type 2 as another subtype is caused by the absence or dysfunction of the mitochondrial membrane carrier protein (SLC25A13), also called CITRIN. Pseudodendritic keratitis is a rare condition that may be seen with tyrosinemia type 2. The association of this ocular complaint with citrullinemia has not been described previously. Awareness of this phenomenon may improve the diagnosis and management of citrullinemia patients.
Keywords: Citrullinemia, Pseudodendritic keratitis, Tyrosinemia
1. Introduction
Citrullinemia is a term used to describe two distinct genetic abnormalities of the urea cycle. Citrullinemia type 1 (CTLN1, OMIM: 215700) results in an accumulation of citrulline in the body fluids as a result of a mutation in the ASS1 gene and a corresponding lack of the argininosuccinate enzyme.1,2 Citrullinemia type 2 (CTLN2, OMIM: 605814) is a different subtype of urea cycle disease (UCD) brought on by mutations in SLC25A13, which impair citrin, an amino acid transporter.3
Despite the fact that CTLN1 can manifest at any age, the classic form of the disease has an early neonatal onset and is first marked by irritability, vomiting, lethargy, and unwillingness to eat. Seizures, hypotonia, respiratory distress, and liver failure may also occur. However, in some patients, particularly those with partial enzyme deficiency, the condition may not manifest until later in infancy or childhood. Failure to flourish, avoiding diets high in protein, ataxia, progressive lethargy, and vomiting are just a few symptoms that may appear.
Infants with the mild variety may experience wellness and hyperammonemia spells alternatively. Children and infants with this form of CTLN1 may experience life-threatening complications, such as hyperammonemic coma.2,3 Due to elevated levels of ammonia in the CSF, CTLN1 could develop into a coma if left untreated (hyperammonemic coma). Infants who are in a hyperammonemic coma for longer than three days may experience neurological abnormalities such as developmental delay, intellectual disability, and cerebral palsy, which are more severe in these cases. Increased muscular tone, spasticity, abnormal reflex movements of the foot, and seizures are possible consequences of elevated intracranial pressure.4
Neonatal intrahepatic cholestasis (NICCD) and recurrent encephalopathy with hyperammonemia and other neuropsychiatric manifestations in adulthood following recovery, are the hallmarks of CTLN2.3
Until now, only 17 occurrences of citrulinemia type 1 have been reported in Iran,5 despite scientific sources estimating that the incidence of UCD is one in 44,300–250,000 (5) and CTLN1 occurs in roughly 1/57,000 births.4 Contrarily, there are widespread mutations in the East Asian population, and the incidence rate of CTLN2 is 1/100,000 to 1/230,000. However, no report of CTLN2 has been published in Iran.6
So far, there is no report of ocular manifestations in any subtypes of citrullinemia or any other subtypes of UCD(s). Here, we report a case of bilateral pseudo-dendritic keratitis in a young patient with normal tyrosine level. After conducting a literature review on PubMed and Google Scholar, using the key words citrulinemia, cornea and keratitis, we did not find any prior reports of citrullinemia as a metabolic disease causing tyrosinemia-like ocular involvement.
2. Case presentation
2.1. Metabolic presentation
A 15 year old female who was admitted to Ali Asghar Children's Hospital in Tehran, Iran, with symptoms of vomiting and stupor. In preliminary investigations, Herpetic encephalitis was suspected based on progressive loss of consciousness, frequent abnormal movement (dystonia), seizure, and slow activity and paroxysmal discharge in Electroencephalography (EEG), where she was treated with antiviral medications. After three days of treatment, her consciousness deteriorated and she was intubated.
Following herpes PCR negative results in plasma and cerebrospinal fluid (CSF), the neurologist suspected autoimmune encephalitis and acute disseminated encephalomyelitis (ADEM). This diagnosis was reinforced by symmetrical signal intensity changes in the thalamus, and treatment with methyl prednisolone was initiated.
Seeing no improvements in consciousness, metabolic disorders were also considered. Ammonia level increase (500 micM/L) and history of two seizures 6 months and 3 years prior to admission, accompanied by delayed development and consanguinity of parents, were important clues for metabolic diagnosis. Citrulinemia was discovered through metabolic testing. After a few days of treatment with ammonia scavengers, her consciousness improved and she was extubated. Later genetic studies revealed mutations in both ASS1 and SLC25A13 genes.
Due to different mutations in type I and II citrullinemia, protein restrictions and enriched carbohydrate diet was prescribed which resulted in health improvement and diminished hyperammonemia. However, due to unpleasant smell and taste of UCD formulas, the patient abstained from consuming this formula and therefore the citrulline level was maintained 4 to 8 times higher than normal range.
2.2. Ophthalmic presentation
Two years after the first presentation, the patient was re-admitted with complaints about bilateral photophobia and tearing. Previously, treatment for suspected allergic conjunctivitis had failed with little success.
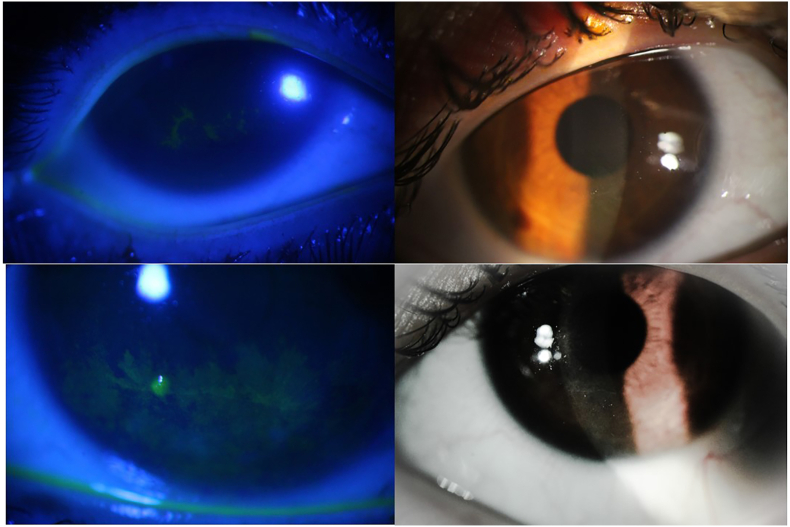
Biomicroscopic examination revealed bilateral corneal haziness with dendritic lesions on the paracentral inferior quadrant. In contrast to HSV dendrites, these lesions were smaller in size, lacked central ulceration and terminal bulbs, implying that they were pseudodendrites. There was no congestion or discharge in either eye (Fig. 1). Other ocular examinations were within normal limits.
Fig. 1.
Corneal pseudodendritic lesions in the patient, with and without fluorescein staining.
Presence of bilateral pseudo-dendrites that remained unresponsive to common medications as well as a history of unresponsiveness to acyclovir, led us to suspect her previous metabolic disease as the cause. All chromatography results showed normal tyrosine levels.
In light of this, it was decided to base the patient's primary treatment on lowering citrulline after consulting with the patient's ophthalmologist and endocrinologist. The patient's photophobia was also managed by using bandage contact lenses and lubricants in tandem.
After three months, her corneal lesions subsided and the symptoms of eye irritation were reduced with a focus on protein restriction and the use of UCD formula. Her mother observed a noteworthy connection between the increase in citrulline levels and the concurrent rise in eye irritation, as well as the decrease in concentration and consciousness due to hyperammonemia. This brought attention to her dietary habits.
2.3. Genetic findings
Genetic study using whole exome sequencing was done and remarked interesting result; there were two important heterozygous variants (compound heterozygote) in ASS1 and a homozygous variant SLC25A13.
For the detected missense variant in ASS1 (NM_054012.3; c.1168G > A, p. G390R) computational prediction tools support a deleterious effect (PP3). It has extremely low frequency in gnomAD population databases (PM2). In ASS1 gene, benign missense mutations have low rate and missense mutation is a common mechanism of a disease (PP2). There are several reports in Clinvar which classify this variant as pathogenic (PP5). In addition the clinical and biochemical phenotype are also compatible with Argininosuccinate synthase deficiency in this patient (PP4). Therefore this variant is classified as pathogenic according to the ACMG classification.7
The donor splice site variant in ASS1 (NM_054012.3; c.688+1G > A) is a null variant in a gene where loss of function is a known mechanism of disease (PVS1). It has extremely low frequency in gnomAD population databases (PM2). Furthermore, the clinical and biochemical phenotype are also compatible with Argininosuccinate synthase deficiency in this patient (PP4). Therefore this variant is classified as pathogenic according to the ACMG classification.7 The heterozygous state in parents and homozygous state in the proband was also confirmed by Sanger sequencing.
3. Discussion
Ophthalmologists are familiar with corneal dendrites which are a common finding in Herpes Simplex Virus keratitis. These lesions will clear spontaneously in some cases and usually respond well to antivirals. However, pseudo-dendritic keratitis must be considered specifically if dendrites are bilateral or not responding to antiviral therapy. These lesions tend to be flatter and finer with fewer branches than true dendrites in herpetic keratitis and they lack terminal bulbs. Pseudodendrites have a wide variety of causes including neurotrophic epitheliopathy, healing epithelial abrasions, contact lens wear, recurrent erosion syndrome and some metabolic diseases. The most well-known metabolic disease that can be associated with these manifestations is tyrosinemia. Tyrosinemia is a rare metabolic disorder which should be suspected in infants presenting with bilateral pseudo-dendrites. Tyrosinemia type II is an amino acid disorder caused by deficiency of the enzyme tyrosine aminotransferase with 75% of the cases presenting ocular involvement. Photophobia due to keratoconjunctivitis may be an early presenting manifestation of the disease. Due to high tyrosine levels in the serum, crystalline deposits may form bilateral superficial punctate corneal opacities with a dendritiform pattern. Other ophthalmic signs in these patients may include corneal haze, conjunctival thickening, Peripheral vascularization, strabismus, nystagmus, and cataract. Early detection is crucial since dietary restrictions for tyrosine and phenylalanine can partially or totally reverse ocular symptoms.
Similar to tyrosinemia, our patient's ocular signs and symptoms improved as a result of dietary changes.
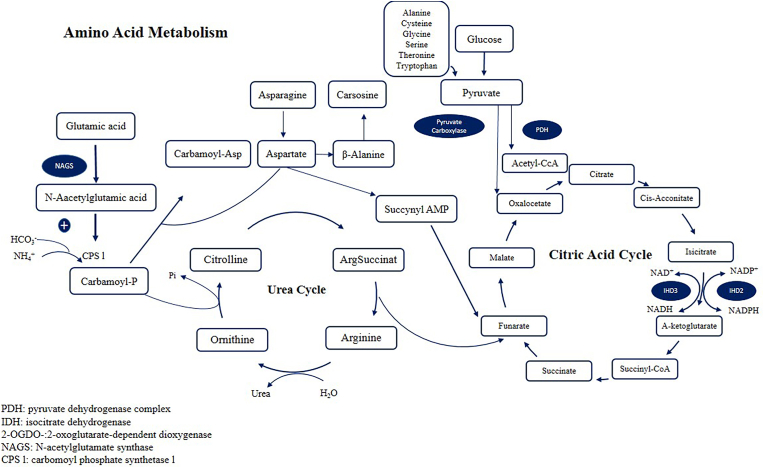
Mitochondrial electrogenic aspartate/glutamate antiporter favors the efflux of aspartate and entry of glutamate and proton into the mitochondria as part of the malate-aspartate shuttle.8 In CTLN2, characteristic alterations in blood amino acid levels are also often observed, including increases in citrulline, arginine, methionine, phenylalanine, tyrosine, and threonine levels, as well as the threonine:serine ratio. Citrin participates in the malate-aspartate shuttle that is linked to the tricarboxylic acid (TCA) cycle, a key pathway in amino acid metabolism. In catabolism, isoleucine, methionine, threonine, and valine are converted to succinyl-CoA, whereas phenylalanine and tyrosine are converted to fumarate, both of which are TCA cycle intermediates. The ketogenic amino acids leucine, lysine, phenylalanine, tryptophan, and isoleucine are converted to acetyl-CoA, entering the TCA cycle. Citrin is also crucial for gluconeogenesis and is required for the degradation of the glucogenic amino acids alanine, glycine, cysteine, serine, aspartate, and asparagine. Furthermore, the breakdown of proline, histidine, arginine, and glutamine produces glutamate, which is a substrate of citrin. Thus, in citrin deficiency, some deregulation of amino acid metabolism including that of tyrosine might occur in all stages.9 The Urea cycle is shown in Fig. 2 (Fig. 2).
Fig. 2.
Urea cycle.
Type 1 citrullinemia is also a urea cycle disorder, and the urea cycle is one of the most important pathways in amino acid metabolism including tyrosine. This cycle is also directly linked to gluconeogenesis.10
One proposed hypothesis regarding the patient's clinical ophthalmologic presentation is that the coincidence of citrullinemia type 1 with type 2 may aggravate the dysfunctions in the tyrosine metabolism, leading to the accumulation of tyrosine in the corneal tissue over time. Tyrosine has a very low solubility of less than 0.5 g/liter in water, although it can be affected by variation in pH and other local biochemistry factors. The normal serum tyrosine level in of our patient was not in accordance with this hypothesis. Another hypothesis regards the accumulation of citrulline in the corneal tissue. Citrulline solubility is much higher than tyrosine and about 200 g/liter (at 20C). However we found no previous report of citrulline accumulation in corneal tissue.
Confocal microscopy is a noninvasive diagnostic imaging method that can be used for detecting many pathologies in cornea. Confocal microscopy has been used to confirm the presence of crystals in superficial epithelium in cases with Tyrosinemia. We couldn't perform this imaging on our patient, as she wasn't cooperative enough due to photophobia. However confocal microscopy can only reveal the presence of crystals in corneal tissue and cannot differentiate between tyrosine or citrulline crystals. Pathologic evaluation appears to be the only approach to confirm the diagnosis.
In this study, the variant found on the ASS1 gene was a known pathogenic variant. Therefore, it was predicted that this variant would cause a serious disturbance in the metabolism of citrulline. But the variant found in the SLC25A13 gene was a new variant of the VUS class and its functional significance was unknown. It seems that the SLC25A13 variant may aggravate the abnormalities of citrulline and tyrosine metabolism caused by the ASS1 variant, especially in the tissues where these genes are expressed. These genes are expressed in the corneal epithelial cells.11 However, how much the disruption in the function of these genes due to variants disrupts citrulline and tyrosine metabolism requires functional studies.
Ethics approval and consent to participate
This study adhered to the tenets of the Declaration of Helsinki. The patient was informed about the study goals and informed consent was obtained.
Consent for publication
The patient was informed about publication of her data and informed consent was obtained.
Data availability
All data generated or analyzed during this study are included in this published article.
Funding
The authors declare they have no financial interests.
Authors' contributions
All authors made substantial contributions to conception, providing data and writing the manuscript.
CRediT authorship contribution statement
Davoud Amirkashani: Data curation, Writing – original draft, Writing – review & editing. Saeid Talebi: Conceptualization, Writing – original draft, Writing – review & editing. Ali Zekri: Conceptualization, Writing – original draft, Writing – review & editing. Parisa Abdi: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Mahdokht Mehramiz: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors have no conflict of interest.
Aknowledgements
Not applicable.
Contributor Information
Davoud Amirkashani, Email: damirkashani58@gmail.com.
Saeid Talebi, Email: talebisaid@gmail.com.
Mohammad Vafaei shahi, Email: dr.vafaeeshahi@yahoo.com.
Ali Zekri, Email: azekri87@gmail.com.
Parisa Abdi, Email: pabdi@sina.tums.ac.ir.
Mahdokht Mehramiz, Email: mahdokht68@gmail.com.
References
- 1.Margulies L.J., Mannis M.J. Dendritic corneal lesions associated with soft contact lens wear. Arch Ophthalmol. 1983;101(10):1551–1553. doi: 10.1001/archopht.1983.01040020553009. [DOI] [PubMed] [Google Scholar]
- 2.Charlton K.H., Binder P.S., Wozniak L., Digby D.J. Pseudodendritic keratitis and systemic tyrosinemia. Ophthalmology. 1981;88(4):355–360. doi: 10.1016/s0161-6420(81)35036-2. [DOI] [PubMed] [Google Scholar]
- 3.Macsai M.S., Schwartz T.L., Hinkle D., Hummel M.B., Mulhern M.G., Rootman D. Tyrosinemia type II: nine cases of ocular signs and symptoms. Am J Ophthalmol. 2001;132(4):522–527. doi: 10.1016/s0002-9394(01)01160-6. [DOI] [PubMed] [Google Scholar]
- 4.Gokhale N.S., Dherai A.J., Desai H., Ashavaid T.F. Unusual dendritic keratitis. Indian J Ophthalmol. 2007;55(1):57–59. doi: 10.4103/0301-4738.29497. [DOI] [PubMed] [Google Scholar]
- 5.Goldsmith L.A. Tyrosinemia II: lessons in molecular pathophysiology. Pediatr Dermatol. 1983;1(1):25–34. doi: 10.1111/j.1525-1470.1983.tb01088.x. [DOI] [PubMed] [Google Scholar]
- 6.Kocabeyoglu S., Mocan M.C., Irkec M. In vivo confocal microscopic features of corneal pseudodendritic lesions in tyrosinemia type II. Cornea. 2014;33(10):1106–1108. doi: 10.1097/ICO.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 7.Richards S., Aziz N., Bale S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmieri L., Pardo B., Lasorsa F.M., et al. Citrin and aralar1 are Ca(2+)-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001;20(18):5060–5069. doi: 10.1093/emboj/20.18.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavoulari S., Lacabanne D., Thangaratnarajah C., Kunji E.R.S. Pathogenic variants of the mitochondrial aspartate/glutamate carrier causing citrin deficiency. Trends Endocrinol Metab. 2022;33(8):539–553. doi: 10.1016/j.tem.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watford M. The urea cycle: teaching intermediary metabolism in a physiological setting. Biochem Mol Biol Educ. 2003;31(5):289–297. [Google Scholar]
- 11.van Zyl T., Yan W., McAdams A.M., Monavarfeshani A., Hageman G.S., Sanes J.R. Cell atlas of the human ocular anterior segment: tissue-specific and shared cell types. Proc Natl Acad Sci U S A. 2022;119(29) doi: 10.1073/pnas.2200914119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.