Figure-5: Sperm fertilization parameters.
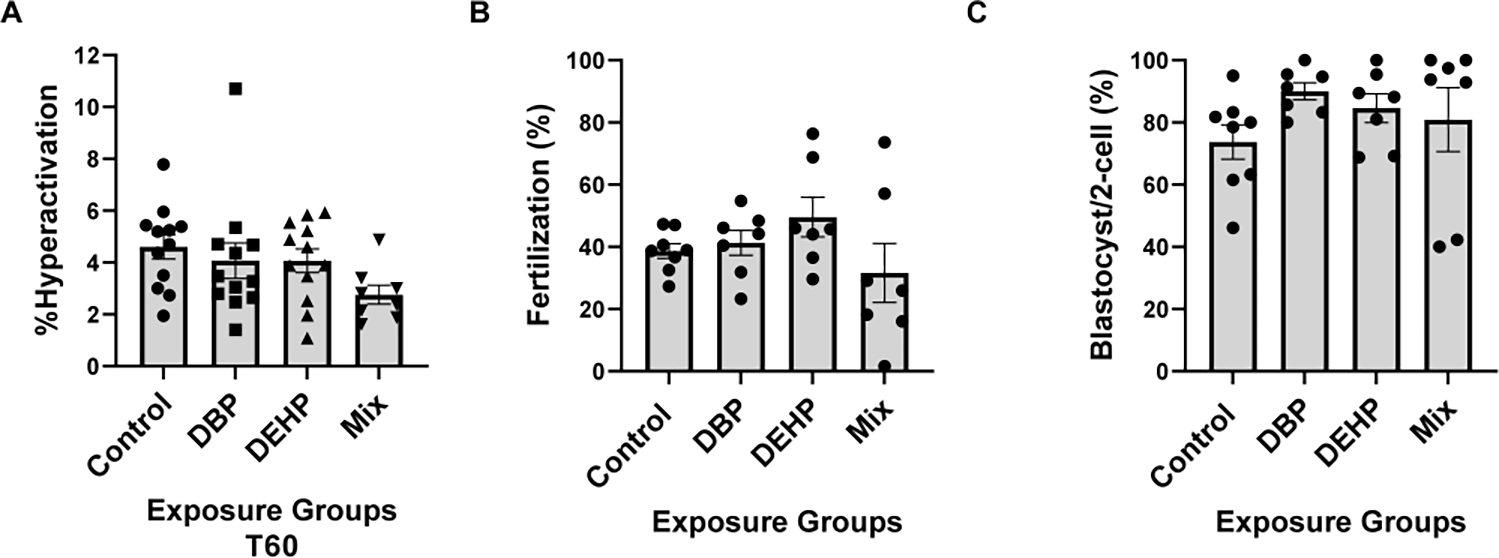
A. Sperm hyperactivation was measured using CASA with the same samples obtained for the analyses depicted in Fig. 1; those spermatozoa with VCL > 271.00 μm/sec, LIN<50.00% and ALH>3.50μm were considered to be hyperactive. A one-way Anova was performed followed by Dunnett test for multiple comparisons using GraphPad prism with a sample size of N=12. Data are represented as mean percentage of abnormal sperm ± SEM (N=12). B. Epididymal sperm were obtained from mice exposed to control or to phthalates and capacitated for 1 hour in complete TYH media. Approximately 1 × 105 sperm were added to the inseminaton droplet. Fertilization was calculated as percentage of oocytes capable of reaching two-cell embryo stage after heterologous fertilization between CD-1 female oocytes and phthalate exposed C57BL/6J sperm from each group (control, DBP, DEHP, Mix), respectively. Kruskal-Wallis non-parametric test was performed followed by Dunn’s test using Graph Pad Prism software to compare within groups with a sample size of N=7. C. Embryo development rates. Two-cell embryos were incubated in KSOM media for 3.75 days up-to-blastocyst stage. Data are expressed as percentage of two-cell embryos from B that reached blastocyst stage. For each of the fertilization assays Kruskal-Wallis non-parametric test was performed followed by Dunn’s test for multiple comparisons using GraphPad Prism software to compare within groups (N=7) at p<0.05.
