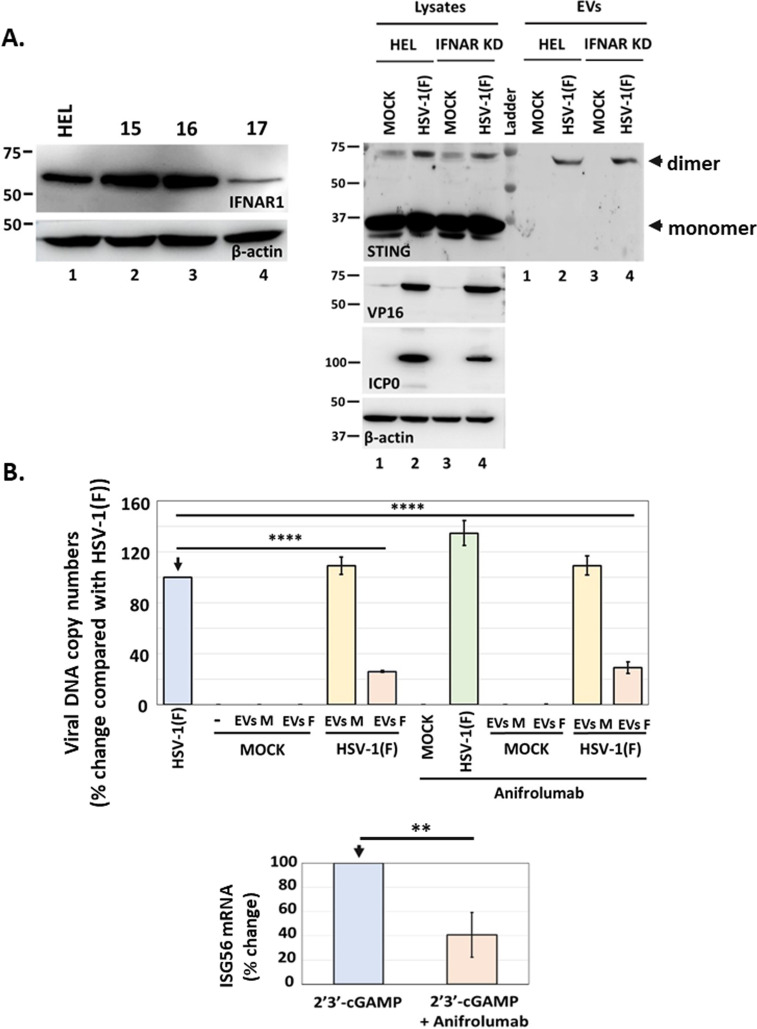
Fig 9.
Effect of IFNAR1 on STING exocytosis and the antiviral function of EVs. (A) HEL cells and the IFNAR1 KD derivatives were either uninfected or infected with HSV-1(F) (0.5 PFU/cell). Cell lysates and culture supernatants were harvested at 48 h post-infection, and equal amounts of proteins from cell lysates were analyzed for STING expression. EVs were isolated from the supernatant after centrifugation at 100,000 × g. Total EVs were analyzed by western blot for STING. VP16 and ICP0 were used as controls for the infection and β-actin as a loading control. The efficiency of IFNAR1 depletion by different shRNAs expressed from an integrated lentiviral vector following puromycin selection is depicted. More efficient IFNAR1 depletion was achieved by shRNA #17. (B) EVs were produced and isolated from uninfected and HSV-1(F)-infected HEL cells as in Fig. 8A. Replicate cultures of HEL cells were treated or not with anifrolumab (1 µg/mL), an IFNAR1 neutralizing for 2 h. The cells were then treated or not with EVs from uninfected (3,000 EVs/cell) or EVs from HSV-1(F)-infected cells (4,300 EVs/cell) for 3 h. EVs were washed, and the cells were infected or not with HSV-1(F) (0.01 PFU/cell). The cells were harvested at 24 h post-infection and analyzed for viral genome copy numbers as in Fig. 8. As a control, we transfected HEL cells with 2′3′-cGAMP (6 µM) in the presence or absence of anifrolumab (1 µg/mL). Cells were harvested at 24 h post-treatment, and ISG56 mRNA was quantified by RT-qPCR.