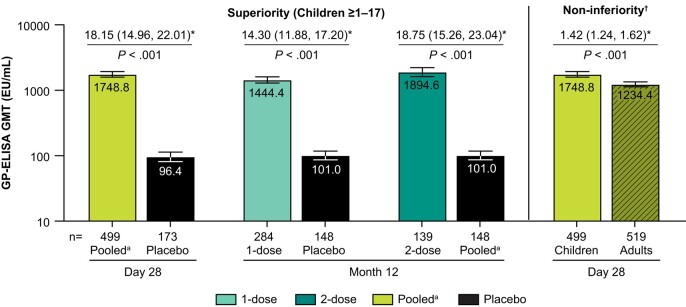
Figure 2.
Pre-specified primary immunogenicity objectives. The bars represent GP-ELISA GMT (EU/mL) with 95% CI for the pooled arm at Day 28 (post-dose 1), and at Month 12 for the 1- and 2-dose arms, compared to placebo. GMTs from children are shown in the solid bars and GMTs from adults are shown in the hatched bars. *Estimated fold difference with 95% CI and results of hypothesis testing (superiority and non-inferiority) are shown above each bar. n = number of participants contributing to the analysis. †Non-inferiority margin =0.5. aPooled = 1- and 2-dose rVSVΔG-ZEBOV-GP arms, post-dose 1. Abbreviations: CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; GMT, geometric mean titer.