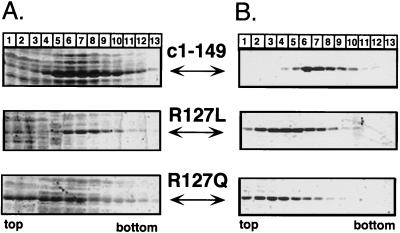
FIG. 7.
Sedimentation analysis of R127 variants. (A) Analytical sucrose gradients of crude E. coli lysates. Aliquots of crude lysates from bacteria transformed with the appropriate expression plasmids encoding proteins c1–149 and its R127 variants were subjected to sedimentation in 10 to 60% sucrose gradients without prior ammonium sulfate precipitation. Aliquots of each fraction were analyzed by SDS-PAGE and Coomassie blue staining. R127L sedimented essentially to the same central fractions as did wild-type protein c1–149, while most of protein R127Q was present in the top fractions. (B) Reassociation of isolated dimers. Variants R127L and R127Q, purified as dimers by size exclusion chromatography, were subjected to reassociation conditions, in parallel with isolated c1–149 dimers (see Materials and Methods for details), and the reaction products were analyzed on analytical sucrose gradients. These conditions led to efficient reassociation of the wild-type protein (top), while only small amounts of the variant proteins were detected in particle-specific fractions.