Figure 1.
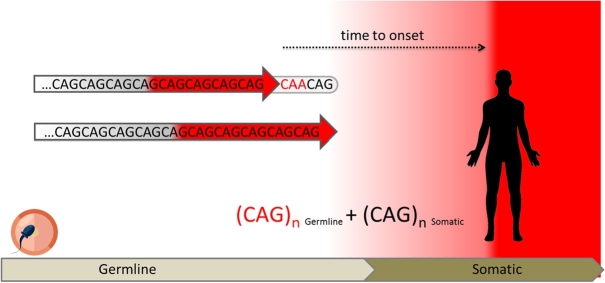
Interplay of inherited and somatic expansions to the progression of HD. The length of the HTT CAG repeat determines the disease phenotype. The disease occurs in individuals who have inherited a repeat tract that has expanded beyond a certain length. Individuals who carry an expanded HTT allele can become symptomatic at any time point in their life and be healthy until then with no apparent signs of the disease. The normal range of CAG repeats is present in unaffected individuals (CAG on a gray background). HTT alleles expanded into the pathological range (CAG on a red background) lead invariably to the onset of HD, and longer alleles are usually associated with an earlier onset of the disease. Although people with reduced-penetrance (RP) alleles have the chance of being asymptomatic throughout lifetime, there are always cases that become symptomatic earlier than expected. Some of these HD patients carry a loss of the penultimate glutamine-encoding CAA codon (in red) in their HTT allele. They have identical polyglutamine tract lengths as subjects with the frequent CAA codon, but exhibit a longer uninterrupted CAG sequence (red arrow), thus indicating that the pure number of uninterrupted CAG repeats (red arrow) makes a more decisive contribution to AO of HD than the number of encoded polyglutamines. Cis- and trans-acting modifiers like the loss of the CAA repeat interruption or variations in DNA repair genes appear to significantly affect the rate at which somatic expansions occur. Under the assumption that the repeat undergoes somatic expansion throughout lifetime, brain cells being most susceptible to disease pathogenesis in particular, HD becomes manifest and the first symptoms appear when the repeat length (the sum of the inherited and somatic expansions, CAGn germline + CAGn somatic) exceeds a disease-specific threshold that may determine the onset of overt toxicity.