Key Points
-
•
A relationship between low baseline DOAC levels and thrombotic events in 1-year follow-up was found.
-
•
Early measurement allows the identification of patients with low DOAC levels and treatment adjustment to avoid future thrombotic events.
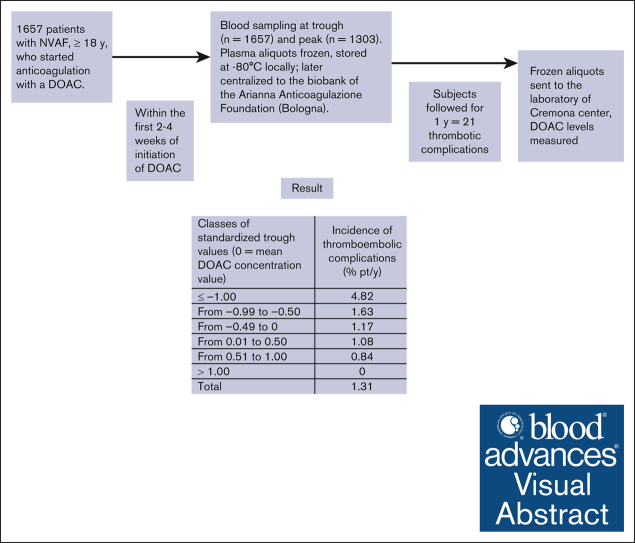
Visual Abstract
Abstract
Although effective and safe, treatment with direct oral anticoagulants (DOAC) in atrial fibrillation (AF) is still associated with thrombotic complications. Whether the measurement of DOAC levels may improve treatment efficacy is an open issue. We carried out the observational, prospective, multicenter Measure and See (MAS) study. Blood was collected 15 to 30 days after starting DOAC treatment in patients with AF who were followed-up for 1 year. Plasma samples were centralized for DOAC level measurement. Patients’ DOAC levels were converted into drug/dosage standardized values to allow a pooled analysis in a time-dependent, competitive-risk model. The measured values were transformed into standardized values (representing the distance of each value from the overall mean) by subtracting the DOAC-specific mean value from the original values and dividing by the standard deviation. Trough and peak DOAC levels were assessed in 1657 and 1303 patients, respectively. In total, 21 thrombotic complications were recorded during 1606 years of follow-up (incidence of 1.31% of patients per year). Of 21 thrombotic events, 17 occurred in patients whose standardized activity levels were below the mean of each DOAC (0); the incidence was the highest (4.82% of patients per year) in patients whose standardized values were in the lowest class (−1.00 or less). Early measurement of DOAC levels in patients with AF allowed us to identify most of the patients who, having low baseline DOAC levels, subsequently developed thrombotic complications. Further studies are warranted to assess whether thrombotic complications may be reduced by measuring baseline DOAC levels and modifying treatment when indicated. This trial was registered at www.ClinicalTrials.gov as #NCT03803579.
Introduction
Over the last years, clinical trials, meta-analyses, and clinical practice confirmed the efficacy and safety of direct oral anticoagulants (DOACs) for stroke prevention in patients with nonvalvular atrial fibrillation (AF).1, 2, 3, 4, 5, 6, 7, 8 Recent observational studies in patients with AF showed that DOACs, compared with warfarin, had lower rates of stroke, systemic embolism, comparable rates of major bleedings,9 and advantages in terms of risk reduction of intracranial bleeding and systemic embolism, even in older and frail populations.10,11 However, a nonnegligible incidence of thrombotic and bleeding events has been recorded in clinical trials and in observational studies in patients receiving DOAC.5,7,8 Hence, the issue of improving the clinical management of patients treated with DOACs and further reducing the risk of complications during treatment is relevant.
DOACs are administered to patients with AF based on patient characteristics such as age, comorbidity, body weight, and renal function without dose adjustment based on DOAC concentration measurements. This is mainly based on results of pharmacokinetic studies, which indicated a predictable anticoagulant response and an effective prevention of excessive drug concentration.12, 13, 14, 15, 16 Furthermore, the registration trials were conducted at dose regimens adjusted for some patient characteristics or for concomitant use of associated interacting drugs, and not for measured DOAC levels.
Consequently, the measurement of DOAC levels has been recommended only in particular situations, such as bleeding or thrombotic complications, before urgent need of surgery or invasive procedures, use of antidotes, and also suggested in special patient populations, such as those with frailty, those who are underweight or overweight, or treated for epilepsy.17, 18, 19 However, studies focusing on the measurement of plasma DOAC levels showed high interpatient variability for all the DOACs and for all the doses used to treat patients.20, 21, 22, 23, 24
Moreover, postmarketing studies showed that the variability of DOAC levels was even higher than those reported in phase 2 and 3 studies.25, 26, 27, 28, 29 Indeed, observational studies reported that some of the patients treated at fixed doses may have relatively high or low DOAC plasma levels, thus supporting the issue of assessing whether an early or periodical measurement might contribute to improving the quality of treatment and reducing risks of complications.30,31
In addition, very recent studies showed a relationship between low DOAC levels (generally measured after the events), the risk of ischemic stroke and its severity,32 and the risk of stroke recurrences.33 Finally, a pilot prospective multicenter study evidenced low plasma levels of DOACs in patients with AF, measured at the time of embolic stroke.34 Whether DOAC concentration measurement may be useful to better tailoring the dose and optimizing the risk-benefit of treatment remains an unsolved clinical problem.
The present study aimed to investigate whether low DOAC plasma levels, assessed at steady-state within the first month of treatment, are associated with thrombotic events during a 1-year follow-up.
Material and methods
The Measure and See study (MAS; ClinicalTrials.gov identifier: NCT03803579) is an observational, prospective cohort, multicenter study of patients with AF, who started treatment with 1 of the available DOACs (dabigatran, apixaban, edoxaban, and rivaroxaban) for therapy and prevention of thrombotic complications. The study was promoted and funded by the Arianna Anticoagulazione Foundation (Bologna, Italy) and conducted in anticoagulation clinics affiliated with the Italian Federation of Anticoagulation Centers.
Patient population
Consecutive patients with AF, without rheumatic mitral valve disease or mechanical heart valves, aged >18 years, seen at the anticoagulation clinics from 27 August 2018 to 10 November 2022, who had started anticoagulation with a DOAC within 1 month, were enrolled in the study. Patients suitable for electrical cardioversion, those who had refused blood sampling, those who did not accept follow-up for at least 1 year, and those who had other clinical indications for anticoagulant therapy were excluded from the study.
The choice of DOAC and dose used was left to the discretion of treating physicians. The study protocol included a recommendation for the participant centers to include patients who were treated following the rules defined for each drug by the Italian regulatory medicines agency (Agenzia Italiana del Farmaco) and current clinical practice.
Each patient was given a unique anonymous identifying code to ensure anonymity, which was used to collect clinical information and identify biological samples. The following baseline characteristics were recorded in a specific electronic database: patient identification number, date of birth, sex, type of drug used and dose, weight, body mass index (BMI), kidney function (estimated by creatinine clearance according to the Cockroft-Gault formula), liver enzyme function (assessed by aspartate aminotransferase and alanine aminotransferase), diabetes, CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 [doubled], diabetes, stroke [doubled], vascular disease, age 65 to 74 and sex category [female]) score, previous stroke/transient ischemic attack (TIA), other comorbidities, and concomitant medications, (with particular attention to antiplatelet drugs). Data were stored in the database located at a section of the Aruba cloud rented by the Arianna Anticoagulazione Foundation, which guaranteed the database's storage, backup, and maintenance.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. Independent review board approval was obtained before all study-related activity from the ethics committee of the coordination center (Cremona; approval no. 14725; 02/05/2018) and from the ethics committees of all other centers. Written informed consent was obtained from each patient before enrollment. The study promoter provided measures to safeguard patient privacy and the protection of personal data according to the European Union General Data Protection Regulations 2016/679 and Italian law.
Blood sampling and DOAC measurement
The study required a mandatory plasma collection to measure the DOAC level for each enrolled patient. Venous blood sampling had to be performed at a steady state (within the first 2-4 weeks of initiation of treatment) and obtained at the time of trough (C-trough), immediately before the subsequent drug intake. It was up to the discretion of participating centers, depending on their availability and organization, to perform additional blood collection on the same day, 2 hours after the last intake (C-peak). Further blood samples were used to perform ancillary laboratory tests (including blood cell count, creatinine clearance, and liver enzymes). Plasma samples for DOAC measurement were collected in vacuum tubes (Vacutainer; Franklin Lakes, NJ) containing 1-in-10 volumes of 3.2% trisodium citrate. Blood was centrifuged within 1 hour of collection at 2000g for 20 minutes (controlled room temperature), and plasma samples were aliquoted in cryovials, identified locally to maintain patient anonymity, in volumes that allow for optimal DOAC testing; sample vials were stored frozen (−80°C)35 at the participating centers and later centralized to the biobank of the Arianna Anticoagulazione Foundation (Bologna). Finally, aliquots were transferred (dry-ice and express courier) to the Haemostasis and Thrombosis Center of Cremona Hospital for DOAC measurement.
DOAC levels, expressed as drug concentration-equivalent (ng/mL), were measured by chromogenic assays using STA-ECA II (Diagnostica Stago, Asnieres-sur-Seine, France) for dabigatran, and STA-Liquid anti-Xa (Diagnostica Stago) for apixaban, edoxaban, and rivaroxaban (hemolyzed samples were discarded).36, 37, 38 Tests were calibrated using commercial plasmas with certified DOAC concentration supplied by the same manufacturer and performed on STA-R Max instrument (Diagnostica Stago). The results of DOAC levels for each patient were transmitted to the central database repository and were not communicated to patients, participating centers, or attending physicians.
Follow-up and outcomes
Participating centers were instructed to organize blood sampling and clinical follow-up as requested by the study. Follow-up, as defined by Italian Federation of Anticoagulation Centers guidelines, included a clinical evaluation within the first month of treatment and a clinical check-up every 3 to 4 months for 1 year. All thromboembolic and bleeding complications, death, and other events were recorded during the 12-month follow-up.
The predefined study outcomes were all thromboembolic complications, including objectively documented ischemic cerebral vascular events, systemic emboli, the occurrence of acute venous thromboembolism (VTE), acute myocardial infarction, and thrombotic and cardiovascular deaths. An independent adjudication committee, that was unaware of patient name, the results of DOAC levels in the collected samples, and the enrolling center, assessed all of the adverse events occurring during follow-up. This assessment reports analyses of the data regarding the relationship between DOAC levels and the occurrence of thromboembolic complications during follow-up. The relationship between DOAC levels and bleeding events will be analyzed and reported separately.
Statistical analysis
Sample size
Based on previous studies,12,25 we hypothesized that the annualized risk of the primary study outcome may be fourfold higher in the lowest quintile of DOAC distribution than in the highest quintile of DOAC distribution. We assumed an annualized risk of primary study outcome of 1.25% of patients per year (pt/y), and 5% pt/y in patients in the highest and lowest quintile of DOAC plasma concentration, respectively. Under this assumption, a total sample size of 1315 patients would be sufficient to refute a null hypothesis of equivalence with α and β errors of 0.05 and 0.2, respectively.
Analysis plan
Because the absolute C-trough and C-peak DOAC plasma concentrations are drug- and dose-dependent, we standardized each measured absolute value using their drug- and dose-specific mean and standard deviation. Standardized values represent the distance of each value from the drug mean distribution and may be, therefore, pooled to evaluate the effect of drug levels, irrespective of the DOAC type and administration (once or twice a day). Supplemental Figure 1 shows the correlation between unstandardized (absolute) and standardized plasma drug concentrations.
The outcome incidence rates were computed for all patients with at least 1 measured plasma DOAC concentration (C-trough or C-peak). Patients were censored at the end of the study or after the occurrence of a qualifying event. For the primary analysis, we used a Cox regression model allowing for competing risk according to the method by Fine and Gray39 to model the occurrence of the primary study outcomes as a function of standardized drug concentration as a continuous value. The regression model included as possible confounders CHA2DS2VASc score, BMI, creatinine clearance, concomitant antiplatelet use, use of low-dose DOAC, and enrolling center. Deaths occurring for causes other than thromboembolic events were considered as competing events. Akaike information criteria was used to evaluate the goodness of fit of the Fine and Gray model. As a sensitivity analysis, we also explored the risk of the primary study outcomes in patients with values below the first quintile of the absolute drug concentration, after adjustment for the aforementioned possible confounders.
As an exploratory analysis, we evaluated the incidence of the primary thrombotic outcome stratified by standardized values. For this analysis, Kaplan-Meier survival curves were plotted to estimate the cumulative incidence of thrombotic outcomes in patients with standardized values in the lowest class compared with those in the higher classes, and hazard ratios and their 95% confidence intervals were calculated. The variable CHA2DS2VASc score (≥4 vs <4) was arbitrarily categorized according to the median values as cutoff. Data were analyzed with the use of Prism software (version 9.3.1, GraphPad Software Incorporated, San Diego, CA) and SPSS software (version 11.0 SPSS Inc, IBM, Armonk, NY), and R (version 4.3.1, R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of patient population
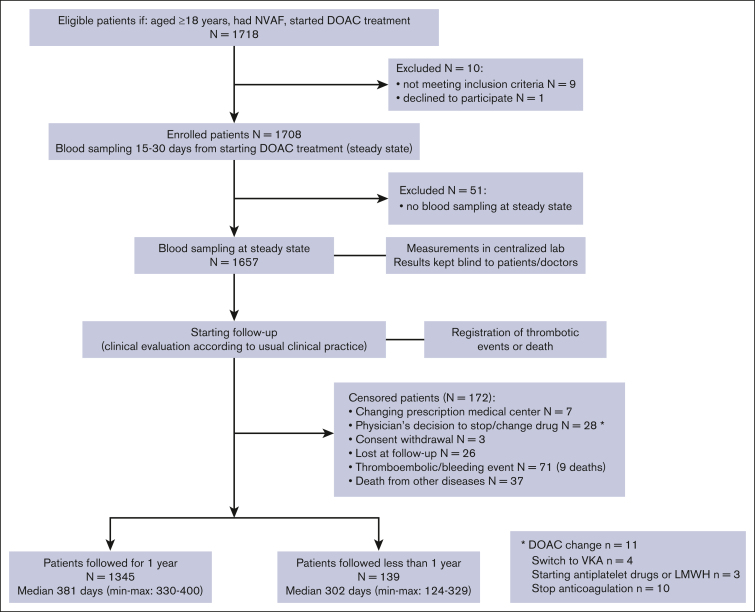
The flowchart of the study population is shown in Figure 1. A total of 1718 patients, who started a DOAC treatment for nonvalvular AF, were included in the study. Ten patients were excluded for not meeting inclusion criteria (n = 9) or declining to participate (n = 1), and 51 were excluded because no blood sampling at steady state was available. A total of 1657 patients had blood sampling for DOAC level measurement, 15 to 30 days from the start of treatment (steady state). The clinical history for 1-year follow-up was collected in 1345 patients and for a shorter period in 139 patients because the study was stopped (median follow-up, 302 days [minimum-maximum: 124-329]). A total of 173 patients were censored before 1 year of follow-up for the following reasons: they had changed the reference medical center (n = 7), the consent to the study was withdrawn (n = 3), the physician decided to change/stop the treatment (n = 28), they were lost to follow-up (n = 26), for thromboembolic or bleeding events (n = 71; including 9 deaths), and death due to other diseases (n = 37). The main demographic and clinical characteristics of the 1657 investigated patients are shown in Table 1.
Figure 1.
Flowchart of the study. NVAF, nonvalvular atrial fibrillation; VKA, vitamin K antagonist.
Table 1.
Baseline characteristics of included patients
| Patients, n | 1657 |
| Participating centers, n | 27 |
| Age, median (min-max), y | 80 (47-100) |
| Males, n (%) | 896 (54.1) |
| BMI, median (min-max) | 26.2 (14.9-68.1) |
| Hemoglobin, median (min-max), g/dL | 13.3 (8.0-18.8) |
| Platelets, median (min-max), X103/μL | 218 (52-700) |
| Creatinine clearance, median (min-max), mL/min | 58.0 (13-246) |
| History of cerebrovascular ischemic disease/peripheral arterial emboli, n (%) | 186 (11.2) |
| History of cardiovascular disease, n (%) | 284 (17.1) |
| History of gastrointestinal bleeding, n (%) | 22 (1.3) |
| History of cancer, n (%) | 227 (13.7) |
| Hypertension, n (%) | 1472 (88.8) |
| Diabetes, n (%) | 375 (22.6) |
| Liver cirrhosis, n (%) | 14 (0.8) |
| Chronic kidney disease, n (%) | 197 (11.9) |
| Hypothyroidism/hyperthyroidism, n (%) | 165 (10.0)/62 (3.7) |
| Smokers, n (%) | 190 (11.5) |
| Alcohol intake, n (%) | 58 (3.5) |
| Mental disorders, n (%) | 52 (3.1) |
| Family/social support, n (%) | 1410 (85.1) |
| Drug daily dose, n (%) | |
| Apixaban (standard dose) | 521 (31.4) [336 (65.5)] |
| Dabigatran (standard dose) | 221 (13.3) [100 (45.3)] |
| Edoxaban (standard dose) | 583 (35.2) [283 (48.6)] |
| Rivaroxaban (standard dose) | 332 (20.0) [238 (71.7)] |
| Prescribing accuracy of DOACs19: | |
| Appropriate, n (%) | 1441 (87.0) |
| Prior VKA treatment, n (%) | 512 (30.9) |
| Use of antiplatelet drugs, n (%) | 382 (23.0) |
| Number of associated drugs, median (min-max) | 3 (0-9) |
| Antihypertensives, n (%) | 933 (56.3) |
| Antiarrhythmics, n (%) | 695 (41.9) |
| Gastroprotectors, n (%) | 655 (39.5) |
| Antidyslipidemics, n (%) | 585 (35.3) |
| Thyroid disease drugs, n (%) | 210 (12.7) |
| Anxiolytics, n (%) | 175 (10.6) |
| Psychotropics, n (%) | 137 (8.3) |
| Painkillers, n (%) | 67 (4.0) |
| Steroids, n (%) | 47 (2.8) |
| Antiepileptic drugs, n (%) | 28 (1.7) |
| Nitrates, n (%) | 13 (0.8) |
| Immunosuppressants, n (%) | 11 (0.7) |
| Antivirals, n (%) | 7 (0.4) |
| Polytherapy ≥3, n (%) | 1196 (72.2) |
| CHA2DS2VASc score, median (min-max) | 4 (0-8) |
| CHA2DS2VASc score ≥4, n (%) | 1072 (64.7) |
min, minimum; max, maximum; VKA, vitamin K antagonist.
Plasma samples for DOAC measurement were available for all patients at C-trough and in 1303 patients at C-peak. Results (mean ± standard deviation; and minimum/maximum) of DOAC levels, at C-trough and at C-peak, are shown in supplemental Table 1, in addition to the number of plasma samples analyzed for the different DOACs.
DOAC concentration levels and thrombotic events during follow-up
During a total follow-up of 1606 years, thromboembolic outcomes occurred in 21 patients (incidence of 1.31% pt/y): 6 strokes (1 fatal), 1 TIA, 1 VTE, 12 acute myocardial infarctions (5 fatal), and 1 superficial vein thrombosis were recorded. Details of patients who had thrombotic outcomes during follow-up are shown in Table 2. Altogether, 46 deaths were recorded (2.8%), 6 of whom were related to thrombotic complications (supplemental Table 2). DOAC plasma level was the most important independent predictor of the occurrence of the primary study outcome according to the best-fitting model (Table 3), even after adjustment for other possible confounders and enrollment centers. Patients with C-peak crude concentrations below the first quintile were particularly at risk of the primary study event (supplemental Table 3).
Table 2.
Details of all thrombotic outcomes
| Sex/age | Thrombotic outcome |
DOAC dose | Weight (kg) | BMI | Creatinine (mg/dL) | Creatinine clearance (mL/min) | C-trough level (ng/mL) | C-peak level (ng/mL) | CHA2DS2VASc score | Previous stroke/TIA | Inappropriate DOAC prescription | Use of antiplatelet drugs | Use of interfering drugs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M/78 | Stroke | Apixaban 5 mg BID | 90 | 24.9 | 1.31 | 69 | 94 | 182 | 4 | Yes | No | No | No |
| F/78 | Stroke | Apixaban 2.5 mg BID | 41 | 18.2 | 1.31 | 23 | 73 | 84 | 7 | Yes | Yes | No | No |
| M/71 | SVT | Apixaban 5 mg BID | 84 | 25.6 | 1.20 | 67 | 110 | 150 | 4 | No | No | Yes | No |
| F/83 | Fatal AMI | Apixaban 2.5 mg BID | 46 | 18.7 | 0.69 | 44 | 33 | 219 | 4 | No | No | No | Yes |
| M/86 | AMI | Apixaban 5 mg BID | 86 | 29.7 | 1.08 | 58 | 106 | 238 | 4 | No | No | No | No |
| M/82 | AMI | Apixaban 5 mg BID | 78 | 26.4 | 1.00 | 63 | 169 | 368 | 4 | No | No | Yes | No |
| M/83 | AMI | Apixaban 2.5 mg BID | 112 | 34.6 | 1.74 | 50 | 97 | NA | 5 | No | No | No | No |
| M/78 | Fatal AMI | Apixaban 5 mg BID | 66 | 23.4 | 1.24 | 45 | 32 | 80 | 6 | No | No | Yes | No |
| M/62 | Stroke | Dabigatran 150 mg BID | 85 | 28.7 | 0.85 | 108 | 68 | 248 | 2 | No | No | No | No |
| M/81 | DVT | Dabigatran 110 mg BID | 73 | 28.5 | 1.27 | 47 | 90 | 165 | 5 | Yes | No | No | No |
| M/67 | AMI | Dabigatran 110 mg BID | 83 | 28.1 | 1.30 | 65 | 37 | 60 | 3 | No | No | Yes | No |
| M/66 | AMI | Dabigatran 150 mg BID | 102 | 32.9 | 1.37 | 75 | 34 | NA | 4 | No | No | Yes | No |
| M/89 | AMI | Dabigatran 110 mg BID | 67 | 21.9 | 0.91 | 51 | 128 | NA | 3 | No | No | No | No |
| F/83 | Stroke | Edoxaban 30 mg | 54 | 23.1 | 0.75 | 48 | 23 | 101 | 5 | Yes | No | No | No |
| F/79 | Stroke | Edoxaban 30 mg | 67 | 26.2 | 0.87 | 55 | 35 | 102 | 5 | No | Yes | No | No |
| M/79 | AMI | Edoxaban 30 mg | 60 | 18.5 | 1.44 | 35 | 20 | 247 | 5 | No | No | Yes | No |
| M/70 | Fatal AMI | Edoxaban 60 mg | 64 | 26.6 | 1.10 | 57 | 40 | 296 | 4 | No | No | Yes | No |
| F/72 | Fatal AMI | Edoxaban 60 mg | 72 | 28.8 | 1.00 | 57 | 52 | NA | 3 | No | No | No | No |
| F/79 | Fatal stroke | Rivaroxaban 15 mg | 55 | 22.9 | 0.60 | 63 | 17 | 121 | 5 | No | Yes | No | No |
| F/89 | TIA | Rivaroxaban 15 mg | 75 | 29.3 | 1.15 | 39 | 13 | 9 | 5 | No | No | Yes | No |
| M/81 | Fatal AMI | Rivaroxaban 20 mg | 104 | 34.0 | 0.57 | 150 | 43 | 189 | 6 | No | No | Yes | No |
AMI, acute myocardial infarction; BID, twice a day (bis in die); DVT, deep vein thrombosis; NA, not available; SVT, superficial vein thrombosis; TIA, transient ischemic attack.
Table 3.
Effect of standardized plasma DOAC levels on the primary thrombotic outcome end point, adjusted for potential confounders
| Characteristic | First model (C-trough), n = 1657 |
Second model (C-peak), n = 1298 |
||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Standardized C-trough DOAC | 0.56 | 0.37-0.86 | - | - |
| Standardized C-peak DOAC | - | - | 0.19 | 0.06-0.66 |
| CHA2DS2VASc score | 2.01 | 1.02-3.97 | 2.07 | 1.26-3.39 |
| BMI, kg/m2 | 0.93 | 0.80-1.08 | 0.95 | 0.82-1.09 |
| Glomerular filtration rate, mL/min | 1.02 | 1.00-1.05 | 1.02 | 0.99-1.05 |
| Low-dose vs standard dose DOAC | 3.49 | 0.76-16.0 | 2.72 | 0.55-13.5 |
| Antiplatelet treatment (yes vs no) | 0.28 | 0.03-2.53 | 0.25 | 0.03-1.81 |
Both models were estimated using the Fine and Gray competitive risk regression model. The Akaike information criteria was 118.4 and 106.1 for the models using C-trough and C-peak, respectively.
The inclusion of enrollment center as a potential confounder was not significant (P > 0.9 for both models) and it is not reported because it did not materially change estimates.
CI, confidence interval; HR, hazard ratio.
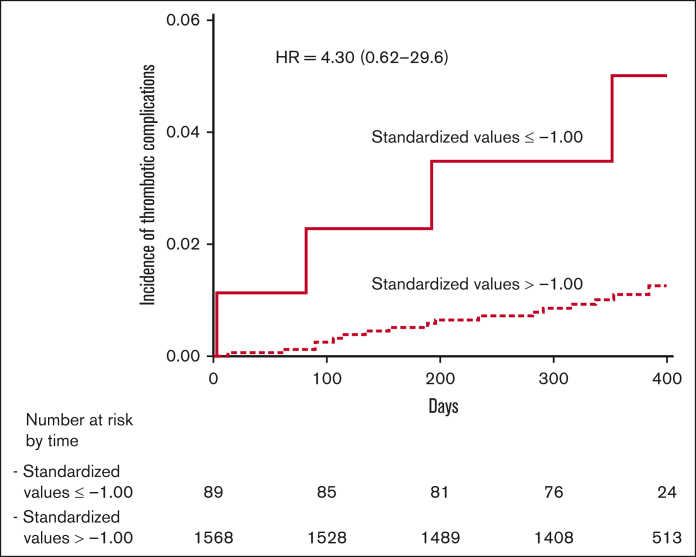
As shown in Figure 2, patients with thrombotic outcomes had C-trough DOAC values below the mean value for each drug in 17 (1.7% pt/y) cases, whereas 4 (0.69% pt/y) cases had values above the mean value. At C-peak, their values were below the mean in 13 (1.8% pt/y) cases and above in 4 (0.70% pt/y) cases. Using standardized C-trough values, patients were distributed into classes of increasing levels (Table 4). The highest incidence of thrombotic events (4.82% pt/y) occurred among 89 patients with standardized DOAC values in the lowest class (−1.00 or less) compared with the mean value; the incidence of events decreased sharply in the other classes. The incidence of events in the lowest class of standardized levels was significantly different than in the sum of all the other classes (4.82% pt/y vs 1.12% pt/y; P = .0039). The Kaplan-Meier curves of cumulative thrombotic outcomes occurring in patients in the lowest class of standardized DOAC levels, assessed at C-trough, compared with those in all the higher classes are shown in Figure 3.
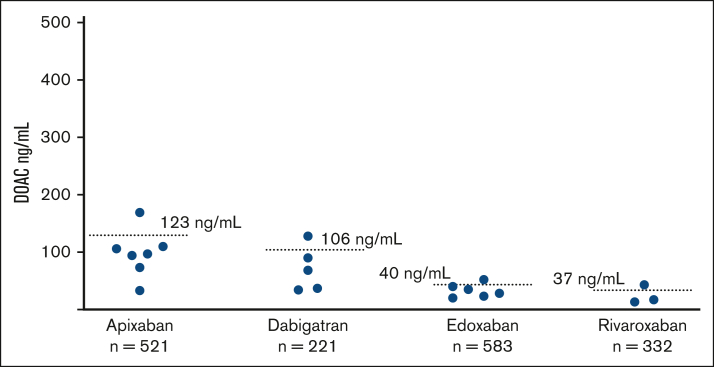
Figure 2.
Measured C-trough DOAC levels assessed at steady-state in atrial fibrillation patients who experienced thrombotic outcomes within 1 year follow-up. Blue dots represent the values and dotted lines represent the mean values of each drug.
Table 4.
Patient distribution in classes of standardized C-trough values around the mean value (0) for all anticoagulant drugs, with the number of patients and of thrombotic complications recorded in each class
| Classes of standardized C-trough values | Equivalent DOAC C-trough plasma levels (ng/mL) |
Patients, n | Follow-up, y | Thrombotic complication, n | Incidence, % pt/y (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Apixaban |
Edoxaban |
Rivaroxaban |
Dabigatran |
||||||||
| 2.5 mg BID | 5 mg BID | 30 mg | 60 mg | 20 mg | 110 mg BID | 150 mg BID | |||||
| -1.00 or less | ≤41 | ≤61 | ≤8 | ≤7 | ≤6 | ≤46 | ≤37 | 89 | 83 | 4 | 4.82 (1.3-12.3) |
| From -0.99 to -0.50 | 44-77 | 63-94 | 9-23 | 8-24 | 8-22 | 51-78 | 38-68 | 442 | 430 | 7 | 1.63 (0.6-3.3) |
| From -0.49 to 0 | 78-113 | 95-128 | 24-39 | 25-42 | 23-37 | 81-109 | 69-99 | 525 | 513 | 6 | 1.17 (0.4-2.5) |
| From 0.01 to 0.50 | 114-146 | 129-160 | 40-54 | 43-58 | 38-52 | 115-138 | 101-128 | 279 | 277 | 3 | 1.08 (0.2-3.2) |
| From 0.51 to 1.00 | 149-179 | 163-195 | 55-70 | 60-73 | 53-67 | 143-163 | 132-163 | 129 | 119 | 1 | 0.84 (0.02-4.7) |
| >1.00 | 187-468 | 199-445 | 72-202 | 79-284 | 70-331 | 175-311 | 165-367 | 193 | 183 | 0 | 0 |
| --- | --- | --- | --- | --- | --- | --- | 1657 | 1605 | 21 | 1.31 (0.8-2.0) | |
The equivalent DOAC plasma levels at C-trough for each class are also reported.
BID, twice a day; CI, confidence interval.
Figure 3.
Cumulative event rates for thrombotic outcomes. The Kaplan-Meier cumulative event rates for the thrombotic outcomes in patients with DOAC levels in the standardized class of −1.00 or less (continuous line) and in patients with DOAC levels in the standardized classes of more than −1.00 (dotted line) at C-trough. HR, hazard ratio.
Discussion
To our knowledge, the MAS study is the first observational, multicenter study that measured DOAC plasma levels in patients with AF at the beginning (steady state) of DOAC treatment who were prospectively followed-up to record thrombotic complications occurring within 1 year. DOAC levels were kept blind to patients and to attending physicians and merged with the individual patients only at the end of the study. The main findings of the study are that 17 of 21 patients who experienced thrombotic outcomes had low plasma activity of the drug at the beginning of treatment; furthermore, the highest incidence of thrombotic complications (4.82% pt/y) occurred in patients whose standardized levels were in the lowest and most distant class from the overall mean.
Efficacy of DOAC treatment for stroke prevention in AF has been widely documented5, 6, 7, 8, 9; however, a nonnegligible incidence of thrombotic events has been recorded in registration trials and clinical practice in patients receiving DOACs.9, 10, 11 These results pose the question of how to improve clinical management of patients with AF treated with DOACs to further reduce the risk of complications. DOAC are administered to patients with AF at fixed dose, either standard or low dose in relation to patient characteristics (mainly age and renal function), without the need for laboratory monitoring. However, high interindividual variability has been confirmed in trials and observational studies,25,27, 28, 29,40, 41, 42 showing that some patients may have low anticoagulant levels and therefore are more exposed to an increased risk of thrombotic complications, especially if at high cardiovascular risk. In a previous study,30 we observed a relationship between low DOAC levels and thrombotic events, particularly in patients with a high CHA2DS2VASc score. Very recently, a monocentric observational study33 reported early stroke recurrence in 3% of patients with AF with a previous stroke, for whom low plasma levels of apixaban and dabigatran had been detected at steady state.
In this study, which involved 1657 patients with AF treated with the 4 DOACs currently on the market, we collected data on thrombotic cardiovascular events occurring for a 1-year follow-up after blood sampling taken at a steady state to measure the DOAC levels. We found that >80% of thrombotic complications occurred in patients with standardized values present in the classes below zero (which is the overall mean of DOAC levels). Interestingly, both C-peak and C-trough standardized DOAC plasma concentrations had similar predictive capability in our study; however, C-trough measurements are generally easier to obtain.
Although these results seem to indicate that measurement of DOAC levels at a steady state in patients with AF may help avoiding most thrombotic complications that may occur during treatment, our results clearly show that it would be unsuitable to measure the drug levels in all patients with AF who start a DOAC treatment to avoid (or reduce) the relatively few thrombotic complications that were recorded during the 1-year follow-up (21 events, 1.31% pt/y). Nevertheless, it appears clinically relevant to try to improve the efficacy of DOAC treatment by further lowering the incidence of severe thrombotic complications, which would seem achievable by adjusting the daily dose or changing the drug in patients at high thrombotic risk who show low DOAC levels when measured at the beginning of treatment. To this aim, we propose that different combinations of patient characteristics might be helpful in identifying a criterion that would allow the minimum number of patients to be tested together with the maximum number of thrombotic events that potentially could be avoided.
Limitations
Our study has limitations. The enrollment was strongly affected by the COVID-19 pandemic, which dramatically blocked many activities at the participating centers. We admit that the study may present problems of generalizability because ∼75% of patients were included in only 1 clinical center (Cremona). The 4 available DOACs are not similarly used in Italy and, consequently, the number of investigated patients was not equal for each drug. Although rivaroxaban tablets should be taken with food to increase absorption (product monograph, Bayer, revision April 2023), in this study blood sampling was performed before the administration of the subsequent DOAC dose, and we are not aware whether patient blood sampling was performed before or after food intake for individual patients; DOAC concentration was measured only once (ie, 15-30 days after the initiation of treatment). We therefore cannot exclude possible intraindividual changes in DOAC levels and problems in adherence to treatment during follow-up. However, a previous study reported that the intraindividual variability of DOAC levels was substantially lower than the interindividual variability.28 In contrast, testing DOACs at different time points during follow-up would have been impractical. However, our study showed an association between very low DOAC levels assessed at the beginning of treatment and occurrence of thrombotic complications during 1-year follow-up, and therefore our results were not influenced by possible DOAC level changes after the time point of measurement. It is well known that association with potent inducer agents of DOAC catabolism (such as antiepileptic drugs) may significantly reduce DOAC concentration.43 The concomitant use of antiepileptic drugs in our study was reported in 28 of the investigated patients, 5 of whom had standardized C-trough values in the lowest class around the overall mean (−1.00 or less), and none of these patients had thrombotic complications during follow-up. All in all, we believe that an accurate analysis of the many factors that may interfere with DOAC concentration would be needed to determine which patients should be tested in future studies. For example, DOAC dosing is still uncertain in some groups of patients, such as those with an extreme body weight. Weight and BMI are important variables in drug distribution and plasma concentration levels, and in individuals with very low or high BMI, DOAC measurements may be considered.19 In our study population, the number of patients with BMI of <18.5 or >50 (criteria suggested by Steffel et al19 to define patients with extreme body weight) were small (39 and 1, respectively). The relatively low number of patients with extreme BMI may have contributed to the nonsignificant result of BMI as predictor of thrombotic outcomes in the competitive risk score analysis (Table 3).
The strengths of the study are its prospective and multicentric design; the centralization of laboratory tests, which avoided interlaboratory variations; and the blindness of all test results to patients and treating physicians, which avoided possible treatment adjustment during follow-up.
Future directions
Although we are aware that the results of this study are not sufficient to modify current clinical practice, we believe that our findings may pave the way to future studies aimed to definitively assess whether measuring DOAC plasma levels at steady state in selected patients may reduce the incidence of thrombotic complications during follow-up in the setting of patients with AF.
Conclusion
In conclusion, our data show a relationship between low DOAC levels measured at steady state and occurrence of stroke, TIA, VTE, and other thrombotic cardiovascular events in patients with AF. The results support the clinical utility of measuring DOAC concentration at the beginning of treatment in special settings of patients with AF. Laboratory measurement at steady state may allow to avoid a persistent treatment at insufficient DOAC concentration in patients who are at a very high thrombotic risk. Our results need to be confirmed and expanded with further clinical studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the MAS Study Group appears in “Appendix.”
Acknowledgments
Becton-Dickinson (Franklin Lakes, NJ) provided test tubes for blood sampling, and Diagnostica Stago (Asnieres-sur-Seine, France) provided reagents to perform DOAC concentration analysis.
The study was promoted and funded by the Arianna Anticoagulazione Foundation, which has received an unrestricted grant from the Fondazione Cassa di Risparmio in Bologna to support the study.
Authorship
Contribution: S.T., G.P., and A. Tosetto conceptualized and designed the study; S.T., G.P., A. Tosetto, and C.L. analyzed and interpreted the data; S.T., G.P., and A. Tosetto drafted the article; S.T., G.P., A. Tosetto, C.L., and A. Tripodi critically revised the article for important intellectual content; O.P., A. Ciampa, D.P., R.M., M.T., P.C., R.C.S., A.M.I., E.D.C., P.P., E.M.F., A. Chistolini, M.d.P.E., and M.M. provided study materials or recruited patients; M.C., C.L., and E.A. provided administrative, technical, or logistic support; M.C., C.L., and C.D. collected and assembled data; and all authors approved the final version of the article.
Footnotes
Raw data and scripts used for analysis are available upon request to the authors at the Center for Open Science, Charlottesville, VA; https://osf.io. Original data are available on request from the author, Cristina Legnani (c.legnani@fondazionearianna.org).
The full-text version of this article contains a data supplement.
Contributor Information
Sophie Testa, Email: sophie.testa@asst-cremona.it.
MAS study group:
Sophie Testa, Claudia Dellanoce, Oriana Paoletti, Rossella Morandini, Maurizio Tala, Antonio Ciampa, Martina Gaeta, Paolo Chiarugi, Monica Casini, Valentina Guerri, Rita Carlotta Santoro, Piergiorgio Iannaccaro, Angela Maria Iannone, Maddalena Campagna, Erica De Candia, Maria Adele Alberelli, Maria Basso, Raimondo De Cristofaro, Leonardo Di Gennaro, Antonietta Ferretti, Silvia Sorrentino, Pasquale Pignatelli, Danilo Menichelli, Daniele Pastori, Mirella Saliola, Elena Maria Faioni, Ilaria Avarello, Cristina Razzari, Antonio Chistolini, Simona Michela Aprile, Cristina Santoro, Alessandra Serrao, Maria del Pilar Esteban, Sergio Ricca, Marco Marietta, Laura Arletti, Valeria Coluccio, Giulia Debbia, Deborah Grisolia, Domizio Serra, Alberto Orselli, Alessandra Pescarollo, Sandra Verna, Patrizia Di Gregorio, Giuseppina Cassetti, Mauro Molteni, Mauro Monelli, Carmelo Paparo, Guido Resani, Nicoletta Di Gregorio, Davide Grassi, Corrado Lodigiani, Elena Banfi, Paola Ferrazzi, Luca Librè, Veronica Pacetti, Clara Sacco, Paolo Bucciarelli, Ida Martinelli, Maria Abbattista, Andrea Artoni, Marco Capecchi, Francesca Gianniello, Barbara Scimeca, Anna Turrini, Francesca Moretta, Giorgio Parise, Ciro Zeccardo, Vittorio Fregoni, Massimo Balboni, Federico Leggio, Daniela Poli, Luigi Ria, Marina Spagnolo, Giovanni Dirienzo, Lavinia Dirienzo, Diana Fuzio, Marco Paolo Donadini, Alessandro Squizzato, Walter Ageno, Giovanna Colombo, Silvia Galliazzo, Andrea Gallo, Eleonora Tamborini Permunian, Alexandra Virano, Anna Falanga, Luca Barcella, Sara Gamba, Teresa Lerede, Anna Maggioni, Laura Russo, Francesca Schieppati, Federica Zunino, Giovanni Barillari, Antonella Bertone, Alessandra Poz, Ugo Venturelli, Giuseppina Serricchio, and Francesca Brevi
Appendix: Clinical centers of the MAS study group (in decreasing order of inclusion)
-
•
Sophie Testa, Claudia Dellanoce, Oriana Paoletti, Rossella Morandini, Maurizio Tala. Centro Emostasi e Trombosi, UUOO Laboratorio Analisi chimico-cliniche e microbiologiche, ASST Cremona, Cremona, Italy (1240).
-
•
Antonio Ciampa, Martina Gaeta. Centro emostasi, UOC Laboratorio Analisi, Ospedale S.G. Moscati, Avellino, Italy (54).
-
•
Paolo Chiarugi, Monica Casini, Valentina Guerri. UO di Analisi chimico cliniche, Azienda Ospedaliero Universitaria Pisana, Pisa, Italy (46).
-
•
Rita Carlotta Santoro, Piergiorgio Iannaccaro. Centro Emostasi e Trombosi, UO Emofilia e Patologie della Coagulazione, Azienda Ospedaliero Universitaria Dulbecco, Catanzaro, Italy (35).
-
•
Angela Maria Iannone, Maddalena Campagna. UOSVD Sezione Trasfusionale, Ospedale Don Tonino Bello, Molfetta, Bari, Italy (29).
-
•
Erica De Candia, Maria Adele Alberelli, Maria Basso, Raimondo De Cristofaro, Leonardo Di Gennaro, Antonietta Ferretti, Silvia Sorrentino. UOSD Malattie Emorragiche e Trombotiche, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy (28).
-
•
Pasquale Pignatelli, Danilo Menichelli, Daniele Pastori, Mirella Saliola. UOC Medicina Interna e Prevenzione dell’Aterosclerosi, Azienda Ospedaliero-Universitaria Policlinico Umberto I, Rome, Italy (28).
-
•
Elena Maria Faioni, Ilaria Avarello, Cristina Razzari. Servizio Immunologia e Medicina Trasfusionale, Ospedale San Paolo, ASST Santi Paolo e Carlo, Milan, Italy (19).
-
•
Antonio Chistolini, Simona Michela Aprile, Cristina Santoro, Alessandra Serrao. UO Medicina Traslazionale e di Precisione, Azienda Ospedaliero-Universitaria Policlinico Umberto I, Rome, Italy (18).
-
•
Maria del Pilar Esteban, Sergio Ricca. UO Laboratorio Analisi, Dipartimento dei Servizi Diagnostici, Ospedale Oglio Po, ASST Cremona, Cremona, Italy (18).
-
•
Marco Marietta, Laura Arletti, Valeria Coluccio, Giulia Debbia, Deborah Grisolia. Struttura Complessa di Ematologia, Policlinico di Modena, Azienda Ospedaliera-Universitaria di Modena, Modena, Italy (18).
-
•
Domizio Serra, Alberto Orselli, Alessandra Pescarollo. Servizio Analisi, Ospedale Evangelico Internazionale, Sede di Castelletto, Genoa, Italy (16).
-
•
Sandra Verna, Patrizia Di Gregorio. Servizio di immunoematologia e medicina trasfusionale, Ospedale “SS. Annunziata,” Chieti, Italy (15).
-
•
Giuseppina Cassetti, Mauro Molteni, Mauro Monelli. Medicina Interna, IRCCS Maugeri Milano, Milan, Italy (14).
-
•
Carmelo Paparo, Guido Resani. Laboratorio Analisi, Ospedale Maggiore Chieri, Turin, Italy (11).
-
•
Nicoletta Di Gregorio, Davide Grassi. UOC Medicina Interna e Nefrologia, Presidio Ospedaliero L'Aquila, L’Aquila, Italy (10).
-
•
Corrado Lodigiani, Elena Banfi, Paola Ferrazzi, Luca Librè, Veronica Pacetti, Clara Sacco. Centro Trombosi e Malattie Emorragiche, Humanitas Research Hospital, Rozzano, Milan, Italy (10).
-
•
Paolo Bucciarelli, Ida Martinelli, Maria Abbattista, Andrea Artoni, Marco Capecchi, Francesca Gianniello, Barbara Scimeca. Centro Emofilia e Trombosi Angelo Bianchi Bonomi, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy (9).
-
•
Anna Turrini, Francesca Moretta, Giorgio Parise, Ciro Zeccardo. Laboratorio Analisi Cliniche e Medicina Trasfusionale, IRCCS Ospedale Sacro Cuore Don Calabria, Negrar, Verona, Italy (6).
-
•
Vittorio Fregoni, Massimo Balboni, Federico Leggio. UOC Medicina Generale, Ospedale di Sondalo, Sondalo, Sondrio, Italy (5).
-
•
Daniela Poli, SOD Malattie Aterotrombotiche, Azienda Ospedaliero-Universitaria Careggi, Florence, Italy (5).
-
•
Luigi Ria, Marina Spagnolo. Centro Emostasi e Trombosi, Medicina Generale e Lungodegenza, Ospedale “Sacro Cuore di Gesù” Gallipoli, Lecce, Italy (5).
-
•
Giovanni Dirienzo, Lavinia Dirienzo, Diana Fuzio. UOSVD Patologia Clinica, Ospedale Della Murgia “Fabio Perinei," Altamura, Bari, Italy (4).
-
•
Marco Paolo Donadini, Alessandro Squizzato, Walter Ageno, Giovanna Colombo, Silvia Galliazzo, Andrea Gallo, Eleonora Tamborini Permunian, Alexandra Virano. SSD Degenza Breve Internistica, Ospedale di Circolo e Fondazione Macchi, ASST Sette Laghi, Varese, Italy (4).
-
•
Anna Falanga, Luca Barcella, Sara Gamba, Teresa Lerede, Anna Maggioni, Laura Russo, Francesca Schieppati, Federica Zunino. Servizio di Immunoematologia e Medicina Trasfusionale, ASST Papa Giovanni XXIII, Bergamo, Italy (4).
-
•
Giovanni Barillari, Antonella Bertone, Alessandra Poz, Ugo Venturelli. Ambulatorio Malattie Emorragiche e Trombotiche, Medicina Trasfusionale di Udine, Presidio Ospedaliero Universitario “Santa Maria della Misericordia,” Udine, Italy (3).
-
•
Giuseppina Serricchio, Francesca Brevi. UOC Patologia Clinica, Presidio Ospedaliero Sant'Anna, ASST Lariana, San Fermo della Battaglia, Como, Italy (3).
Supplementary Material
References
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 2.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 4.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 5.Pan KL, Singer DE, Ovbiagele B, Wu YL, Ahmed MA, Lee M. Effects of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6(7) doi: 10.1161/JAHA.117.005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durand M, Schnitzer ME, Pang M, et al. Comparative effectiveness and safety of direct oral anticoagulants versus vitamin K antagonists in nonvalvular atrial fibrillation: a Canadian multicentre observational cohort study. CMAJ Open. 2020;8(4):E877–E886. doi: 10.9778/cmajo.20200055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SR, Choi EK, Kwon S, et al. Effectiveness and safety of direct oral anticoagulants in relation to temporal changes in their use. Circ Cardiovasc Qual Outcomes. 2020;13(3) doi: 10.1161/CIRCOUTCOMES.119.005894. [DOI] [PubMed] [Google Scholar]
- 8.Carnicelli AP, Hong H, Connolly SJ, et al. Direct oral anticoagulants versus warfarin in patients with atrial fibrillation: patient-level network meta-analyses of randomized clinical trials with interaction testing by age and sex. Circulation. 2022;145(4):242–255. doi: 10.1161/CIRCULATIONAHA.121.056355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49(12):2933–2944. doi: 10.1161/STROKEAHA.118.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poli D, Antonucci E, Ageno W, et al. Oral anticoagulation in very elderly patients with atrial fibrillation: results from the prospective multicenter START2-REGISTER study. PLoS One. 2019;14(5) doi: 10.1371/journal.pone.0216831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison SL, Buckley BJR, Ritchie LA, et al. Oral anticoagulants and outcomes in adults ≥80 years with atrial fibrillation: a global federated health network analysis. J Am Geriatr Soc. 2022;70(8):2386–2392. doi: 10.1111/jgs.17884. [DOI] [PubMed] [Google Scholar]
- 12.Reilly PA, Lehr T, Haertter S, et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy) J Am Coll Cardiol. 2014;63(4):321–328. doi: 10.1016/j.jacc.2013.07.104. [DOI] [PubMed] [Google Scholar]
- 13.Girgis IG, Patel MR, Peters GR, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with non-valvular atrial fibrillation: results from ROCKET AF. J Clin Pharmacol. 2014;54(8):917–927. doi: 10.1002/jcph.288. [DOI] [PubMed] [Google Scholar]
- 14.Zeitouni M, Giczewska A, Lopes RD, et al. Clinical and pharmacological effects of apixaban dose adjustment in the ARISTOTLE trial. J Am Coll Cardiol. 2020;75(10):1145–1155. doi: 10.1016/j.jacc.2019.12.060. [DOI] [PubMed] [Google Scholar]
- 15.Salazar DE, Mendell J, Kastrissios H, et al. Modelling and simulation of edoxaban exposure and response relationships in patients with atrial fibrillation. Thromb Haemost. 2012;107(5):925–936. doi: 10.1160/TH11-08-0566. [DOI] [PubMed] [Google Scholar]
- 16.Ruff CT, Giugliano RP, Braunwald E, et al. Association between edoxaban dose, concentration, anti-factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet. 2015;385(9984):2288–2295. doi: 10.1016/S0140-6736(14)61943-7. [DOI] [PubMed] [Google Scholar]
- 17.Tripodi A. To measure or not to measure direct oral anticoagulants before surgery or invasive procedures. J Thromb Haemost. 2016;14(7):1325–1327. doi: 10.1111/jth.13344. [DOI] [PubMed] [Google Scholar]
- 18.Douxfils J, Adcock DM, Bates SM, et al. Update of the international council for standardization in hematology recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2021;121(8):1008–1020. doi: 10.1055/a-1450-8178. [DOI] [PubMed] [Google Scholar]
- 19.Steffel J, Collins R, Antz M, et al. European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23(10):1612–1676. doi: 10.1093/europace/euab065. [DOI] [PubMed] [Google Scholar]
- 20.Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78(4):412–421. doi: 10.1016/j.clpt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Stangier J, Rathgen K, Staehle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64(3):292–303. doi: 10.1111/j.1365-2125.2007.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet. 2009;48(1):1–22. doi: 10.2165/0003088-200948010-00001. [DOI] [PubMed] [Google Scholar]
- 23.Frost C, Nepal S, Wang J, et al. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor Xa inhibitor, in healthy subjects. Br J Clin Pharmacol. 2013;76(5):776–786. doi: 10.1111/bcp.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parasrampuria DA, Truitt KE. Pharmacokinetics and pharmacodynamics of edoxaban, a non-vitamin K antagonist oral anticoagulant that inhibits clotting factor Xa. Clin Pharmacokinet. 2016;55(6):641–655. doi: 10.1007/s40262-015-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Testa S, Tripodi A, Legnani C, et al. Plasma levels of direct oral anticoagulants in real life patients with atrial fibrillation: results observed in four anticoagulation clinics. Thromb Res. 2016;137:178–183. doi: 10.1016/j.thromres.2015.12.001. 137. [DOI] [PubMed] [Google Scholar]
- 26.Testa S, Dellanoce C, Paoletti O, et al. Edoxaban plasma levels in patients with non-valvular atrial fibrillation: inter and intra-individual variability, correlation with coagulation screening test and renal function. Thromb Res. 2019;175:61–67. doi: 10.1016/j.thromres.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Kampouraki E, Avery P, Biss T, Wynne H, Kamali F. Assessment of exposure to direct oral anticoagulants in elderly hospitalised patients. Br J Haematol. 2021;195(5):790–801. doi: 10.1111/bjh.17899. [DOI] [PubMed] [Google Scholar]
- 28.Toorop MMA, van Rein N, Nierman MC, et al. Inter- and intra-individual concentrations of direct oral anticoagulants: the KIDOAC study. J Thromb Haemost. 2022;20(1):92–103. doi: 10.1111/jth.15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwina AE, Dia N, Dreesen E, et al. Insights into the pharmacokinetics and pharmacodynamics of direct oral anticoagulants in older adults with atrial fibrillation: a structured narrative review. Clin Pharmacokinet. 2023;62(3):351–373. doi: 10.1007/s40262-023-01222-w. [DOI] [PubMed] [Google Scholar]
- 30.Testa S, Paoletti O, Legnani C, et al. Low drug levels and thrombotic complications in high-risk atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2018;16(5):842–848. doi: 10.1111/jth.14001. [DOI] [PubMed] [Google Scholar]
- 31.Testa S, Legnani C, Antonucci E, et al. Drug levels and bleeding complications in atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2019;17(7):1064–1072. doi: 10.1111/jth.14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizos T, Meid AD, Huppertz A, et al. Low exposure to direct oral anticoagulants is associated with ischemic stroke and its severity. J Stroke. 2022;24(1):88–97. doi: 10.5853/jos.2020.04952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siedler G, Macha K, Stoll S, et al. Monitoring of direct oral anticoagulants plasma levels for secondary stroke prevention. J Thromb Haemost. 2022;20(5):1138–1145. doi: 10.1111/jth.15677. [DOI] [PubMed] [Google Scholar]
- 34.Nosáľ V, Petrovičová A, Škorňová I, et al. Plasma levels of direct oral anticoagulants in atrial fibrillation patients at the time of embolic stroke: a pilot prospective multicenter study. Eur J Clin Pharmacol. 2022;78(4):557–564. doi: 10.1007/s00228-022-03280-8. [DOI] [PubMed] [Google Scholar]
- 35.Gosselin RC, Marlar RA. Preanalytical variables in coagulation testing: setting the stage for accurate results. Semin Thromb Hemost. 2019;45(5):433–448. doi: 10.1055/s-0039-1692700. [DOI] [PubMed] [Google Scholar]
- 36.He L, Kochan J, Lin M, Vandell A, Brown K, Depasse F. Determination of edoxaban equivalent concentrations in human plasma by an automated anti-factor Xa chromogenic assay. Thromb Res. 2017;155:121–127. doi: 10.1016/j.thromres.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Cini M, Legnani C, Padrini R, et al. DOAC plasma levels measured by chromogenic anti-Xa assays and HPLC-UV in apixaban- and rivaroxaban-treated patients from the START-Register. Int J Lab Hematol. 2020;42(2):214–222. doi: 10.1111/ijlh.13159. [DOI] [PubMed] [Google Scholar]
- 38.de Fautereau-Vassel A, Mokhtarian A, Mangenot M, et al. Comparisons between diluted thrombin time, ecarin chromogenic assays, and UPLC-MS for plasma level dabigatran quantification: results from DRIVING study. Int J Lab Hematol. 2023;46(1):120-127 doi: 10.1111/ijlh.14166. [DOI] [PubMed] [Google Scholar]
- 39.Fine JP, Gray RJ. A proportional hazards model for the sub distribution of a competing risk. J Am Stat Assoc. 1999;94(446):94496–94509. [Google Scholar]
- 40.Gouin-Thibault I, Delavenne X, Blanchard A, et al. Interindividual variability in dabigatran and rivaroxaban exposure: contribution of ABCB1 genetic polymorphisms and interaction with clarithromycin. J Thromb Haemost. 2017;15(2):273–283. doi: 10.1111/jth.13577. [DOI] [PubMed] [Google Scholar]
- 41.Siguret V, Abdoul J, Delavenne X, et al. Rivaroxaban pharmacodynamics in healthy volunteers evaluated with thrombin generation and the active protein C system: modeling and assessing interindividual variability. J Thromb Haemost. 2019;17(10):1670–1682. doi: 10.1111/jth.14541. [DOI] [PubMed] [Google Scholar]
- 42.Foulon-Pinto G, Lafuente-Lafuente C, Jourdi G, et al. Assessment of DOAC in GEriatrics (ADAGE study): rivaroxaban/apixaban concentrations and thrombin generation profiles in NVAF very elderly patients. Thromb Haemost. 2023;123(4):402–414. doi: 10.1055/a-1981-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sennesael AL, Larock AS, Hainaut P, et al. The impact of strong inducers on direct oral anticoagulant levels. Am J Med. 2021;134(10):1295–1299. doi: 10.1016/j.amjmed.2021.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.