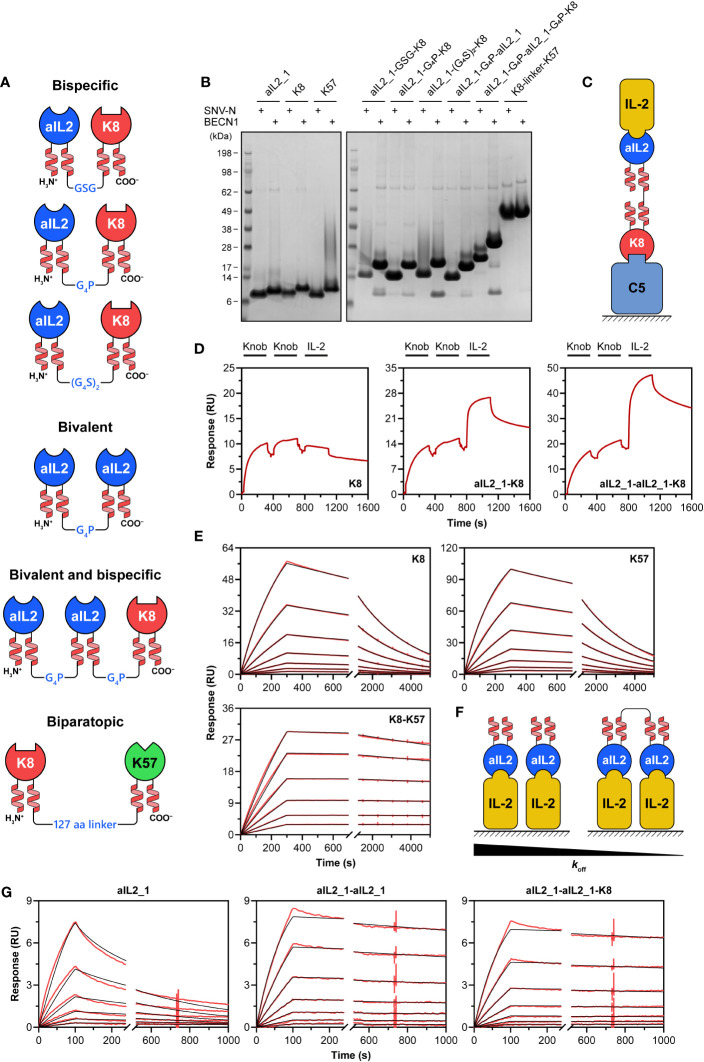
Figure 3.
Bispecific knob domain fusions. (A) Schematic representation of the designed bispecific, bivalent and biparatopic knob domain fusions. (B) SDS-PAGE analysis of knob domain fusions expressed in Expi293F cells. The knob domains were supplied with either SNV-N or BECN1 stalks; individual knob domains were expressed as controls. The gels were run under non-reducing conditions. Migration of K8-K57 fusions (SNV-N, 24 kDa; BECN1, 30 kDa) is slowed down by an extensive glycine-rich linker. (C) Schematic representation of bridging SPR assay used in this study. The surface is coated with one of the antigens (C5), followed by the addition of bispecific fusion. The second antigen (IL-2) is then added. Dissociation of the complex is measured by washing the surface with buffer. (D) The bridging SPR assay confirms simultaneous binding of SNVN-fused bispecific knob domain constructs to their antigens. (E) Representative sensorgrams for binding of SNV-N-fused anti-C5 biparatopic K8-K57 knob domain construct to C5. Individual K8 and K57 were used as controls. Immobilised knob domains were subject to the injections of various concentrations of C5. Experimental curves are shown in red, and the fitted curves are shown in black. (F) Schematic representation of IL-2 binding assay used to confirm simultaneous engagement of two target molecules by bivalent fusions. The surface is coated with an antigen (IL-2), followed by the addition of knob domain constructs. An increase in valency would be associated with a decrease in dissociation rate (koff). (G) Representative sensorgrams for binding of SNV-N-fused bivalent anti-IL-2 constructs to IL-2. Individual IL-2 was used as control. Immobilised IL-2 was subject to the injections of various concentrations of knob domain constructs. Experimental curves are shown in red, and the fitted curves are shown in black.