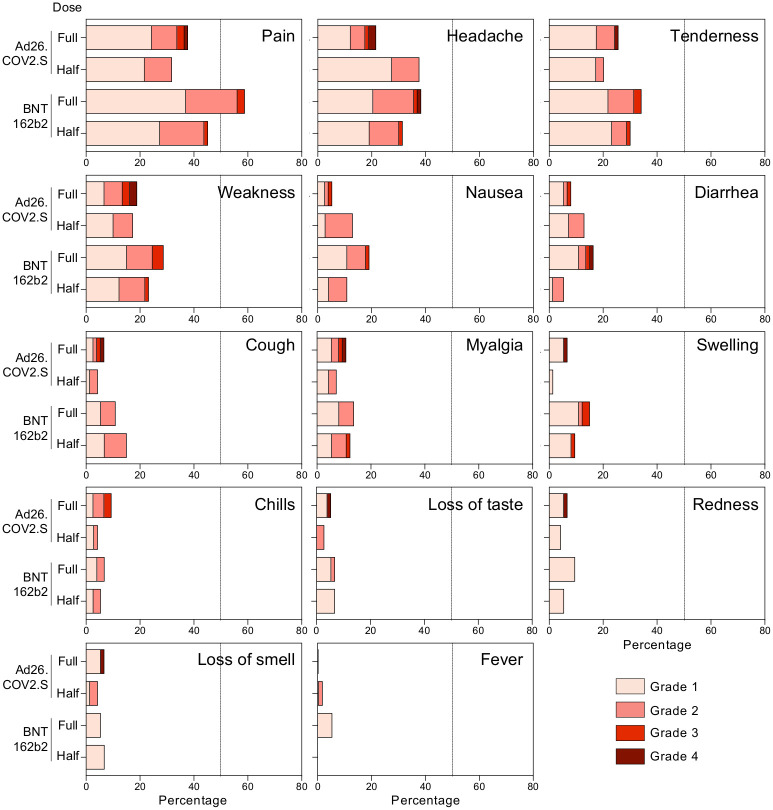
Fig 2. Recorded adverse events in each study arm.
Distribution of participants experiencing adverse events (pain, headache, tenderness, weakness, nausea, diarrhea, cough, myalgia, swelling, chills, loss of taste, redness, loss of smell or fever) recorded 1 week post booster vaccination in each study arm.