Abstract
A 29-year-old man presented with liver damage, and a liver biopsy was performed, but the cause was unclear. Thereafter, he was referred to our hospital. We found that he had been unable to consume carbohydrates in his diet and preferred fried chicken since childhood. In addition, he had shown disturbance of consciousness and abnormal behavior while he had been in prison, where dietary intake had been restricted. A plasma amino acid analysis revealed hypercitrullinemia. Therefore, we suspected adult-onset type II citrullinemia (CTLN2). Genetic testing showed pathologic variations in the SLC25A13 gene, which allowed us to make a definite diagnosis of CTLN2.
Keywords: adult-onset type II citrullinemia, hepatic hypovascular tumor, peculiar eating habits
Introduction
Adult-onset type II citrullinemia (CTLN2) is an autosomal recessive genetic disorder characterized by deficiency of citrin, a hepatic aspartate/glutamate membrane transporter localized at the inner mitochondrial membrane, resulting in impairment in the aspartate supply to the cytosol, mitochondrial NADH supply, and glycogenesis (1). The condition is characterized by various neuropsychiatric symptoms, such as impaired consciousness, disorientation, abnormal behavior, and seizures due to hyperammonemia and hypercitrullinemia. Hepatocellular carcinoma (HCC) at a young age has also been reported (2).
We herein report a case of adult-onset type II citrullinemia that developed under dietary restrictions during imprisonment.
Case Report
A 29-year-old man had been hospitalized for jaundice as a neonate, but the cause had been unclear, and the condition resolved spontaneously. He continued to have liver problems of unknown origin until he reached junior high school and was treated with ursodeoxycholic acid.
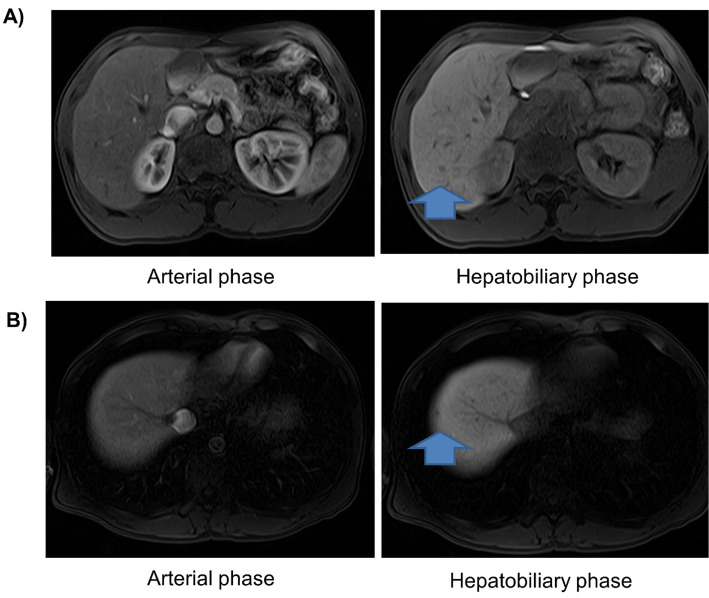
In 2016, he was referred to another hospital for a thorough examination due to liver damage of unknown cause. Various viral hepatitis tests and autoantibody tests were negative, and a liver biopsy was performed, but the cause remained unclear. The patient was treated with ursodeoxycholic acid and glycyrrhizin. Gd-EOB-DTPA-enhanced magnetic resonance imaging (EOB-MRI) performed in March 2020 showed hepatomegaly and hypovascular nodules in S6 and S8, so the patient was referred to our hospital in June 2020 for a close examination. A liver biopsy and MRI showed no evidence of liver steatosis.
His height was 168 cm, and his weight was 58 kg; he was conscious, showed no yellowing of the ocular conjunctiva, and had no abnormal findings in the thorax or abdomen. There were no apparent motor deficits in the extremities. His occupation was in construction. He did not have a history of alcohol consumption. There had been no complications with his mother's pregnancy or his delivery at birth; his birth weight had been 2,670 g, and his height had been 50 cm. As mentioned above, he had been hospitalized for neonatal jaundice. In addition, he underwent appendectomy at 14 years old with no perioperative problems. He did not have a history of acute pancreatitis. Regarding his family history, he lived with his wife and daughter. None of his four siblings had experienced neonatal jaundice or subsequent liver disease. No liver disease had been reported by his parents or other relatives, and his parents were not related by consanguinity.
Blood tests showed that his hepatobiliary enzymes, renal function, and electrolytes were within the standard range, but ammonia was elevated (Table 1). EOB-MRI showed that the liver was enlarged and revealed hypovascular nodules with dimensions of 8 mm at S6 and 5 mm at S8 and without an early contrast effect (Fig. 1). Abdominal ultrasonography showed that the margins of the liver were blunted, and the internal echoes were coarse, whereas it showed no evidence of liver steatosis, such as bright liver. The FibroScan value was 8.3 kPa, and the CAP value was 141.0 dB/m. B-mode and contrast-enhanced imaging with sonazoid administration by ultrasonography did not depict the lesion. Thus, there were few findings to prompt us to actively suspect malignancy, and we diagnosed them as regenerating or dysplastic nodules. Therefore, we followed the patient up with EOB-MRI every six months.
Table 1.
| Hematology | Virus marker | |||||||||
| WBC | 3,100 | /μL | Na | 141 | mEq/L | HBsAg (CLEIA) | 0.0 (-) | C.O.I | ||
| Neut | 31.1 | % | K | 4.3 | mEq/L | anti-HCV (CLEIA) | 0.0 (-) | C.O.I | ||
| Lymph | 53.0 | % | Cl | 104 | mEq/L | |||||
| RBC | 5.57×106 | /μL | CRP | <0.03 | mg/dL | Immunology | ||||
| Hb | 16.0 | g/dL | T-CHO | 146 | mg/dL | IgG | 870 | mg/dL | ||
| Plt | 11.1×104 | /μL | TG | 117 | mg/dL | IgA | 476 | mg/dL | ||
| LDL | 79 | mg/dL | IgM | 96 | mg/dL | |||||
| Biochemistory | Ferritin | 20 | ng/mL | Fe | 101 | ug/dL | ||||
| TP | 7.3 | g/dL | Cu | 71 | ug/dL | AMA-M2 | <1.5 | Index | ||
| Alb | 4.4 | g/dL | Ceruloplasmin | 18 | mg/dL | Anti-smooth muscle antibody | (-) | |||
| AST | 22 | U/L | Zn | 118 | ug/dL | Anti-LKM antibody | <5 | Index | ||
| ALT | 24 | U/L | TSH | 1.330 | uIU/mL | |||||
| LDH | 135 | U/L | FT3 | 3.68 | pg/mL | Hepatic fibrosis and tumor marker | ||||
| ALP | 272 | U/L | FT4 | 0.98 | ng/dL | M2BPGi | 0.47 | C.O.I | ||
| T.Bil | 0.8 | mg/dL | FIB-4 | 1.17 | Index | |||||
| BUN | 21.1 | mg/dL | Coagulation | AFP | 4.5 | ng/mL | ||||
| Cre | 0.83 | mg/dL | PT | 94.6 | % | PIVKA-II | 20 | mAU/mL | ||
| CK | 121 | U/L | PT-INR | 1.04 | CEA | 2.0 | ng/mL | |||
| Glu | 93 | mg/dL | APTT | 30.0 | s | CA19-9 | 12.1 | U/mL | ||
| NH3 | 108 | μg/dL | ||||||||
Figure 1.
EOB-MRI findings at referral. (A) EOB-MRI shows a bloodless nodule with a diameter of 8 mm in S6. (B) EOB-MRI shows a bloodless nodule with a diameter of 3 mm in S8.
After referral, a detailed history of his life and medical history revealed that he had avoided eating carbohydrates, such as rice and udon noodles, since childhood because he vomited when he ate them, and that he had a peculiar dietary history with a preference for proteins, such as fried foods, fish, and cheese. He also avoided juices, preferring to drink milk, and was not a regular alcohol drinker. He had played baseball and soccer beginning in childhood and had no hypoglycemic symptoms. At 25 years old, he had been imprisoned for theft. His intelligence level has been considered to be within the normal range from childhood, and he had not exhibited any neuropsychiatric symptoms by the time he committed his crime. Therefore, his imprisonment was not related to neuropsychiatric symptoms. During his imprisonment, he repeatedly exhibited abnormal behavior, such as nighttime wandering, disturbance of consciousness, disorientation, and incontinence. He was examined by a physician at the time, but the cause was never identified.
After his release from prison, he visited a psychiatrist, where he was diagnosed with panic disorder and temporarily treated with Chinese herbal medicine. However, he subsequently stopped taking the herbal medicine under his own judgment. His psychiatric symptoms spontaneously resolved after his release from prison. Based on the above, CTLN2 was suspected, and a plasma amino acid analysis revealed a high citrulline level of 71.5 nmol/mL (Table 2). To confirm the diagnosis, genetic testing was performed after fully explaining the significance and details to the patient and his family, and pathological mutations in SLC25A13, the gene responsible for citrulline deficiency, were found in a compound heterozygous manner (exon 9, c.852_855del; intron 11, c.1180+ 1G>A), which led to a definite diagnosis of CTLN2. The parents had no history suggestive of CTLN2 and were not related by consanguineous marriage. None of his four siblings had any history suggestive of CTLN2 either; therefore, genetic testing was not performed.
Table 2.
Amino Acid Analysis.
| Citrulline | 71.5 | (17.1-42.6) | nmol/mL | ↑↑ |
| Arginine | 77.8 | (53.6-133.6) | nmol/mL | |
| Ornithine | 62.0 | (31.3-104.7) | nmol/mL | |
| Aspartic acid | 2.0 | (≤2.4) | nmol/mL | |
| Lysine | 186.8 | (108.7-242.2) | nmol/mL | |
| Glycine | 151.1 | (151.0-351.0) | nmol/mL | |
| Alanine | 224.1 | (208.7-522.7) | nmol/mL | |
| Valine | 187.9 | (147.8-307.0) | nmol/mL | |
| Leucine | 108.6 | (76.6-171.3) | nmol/mL | |
| Isoleucine | 44.4 | (43.0-112.8) | nmol/mL | |
| Tyrosine | 55.6 | (40.4-90.3) | nmol/mL | |
| Phenylalanine | 60.4 | (42.6-75.7) | nmol/mL | |
| Tryptophan | 56.2 | (37.0-74.9) | nmol/mL | |
| Methionine | 26.6 | (18.9-40.5) | nmol/mL | |
| Histidine | 82.1 | (59.0-92.0) | nmol/mL | |
| Threonine | 108.1 | (66.5-188.9) | nmol/mL | |
| Serine | 96.6 | (72.4-164.5) | nmol/mL | |
| Cystine | 27.7 | (13.7-28.3) | nmol/mL |
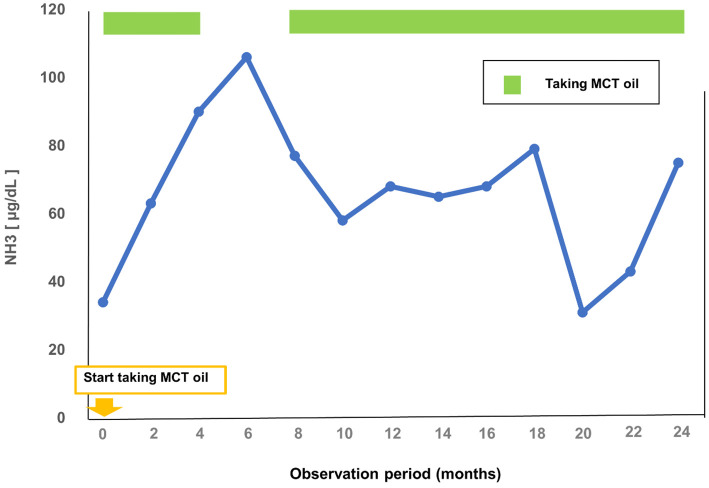
When the patient came to our hospital, his eating habits had not yet been corrected. Ursodeoxycholic acid and glycyrrhizin, which had been prescribed by the previous physician, were continued. He tried eating medium chain triglyceride (MCT) oil, and his ammonia level did not increase when MCT oil was properly taken (Fig. 2). However, after the diagnosis was confirmed by genetic testing, the patient exhibited several episodes of abnormal behavior, such as nighttime wandering and impaired consciousness, and his ammonia level increased. The patient was treated with a fixed amount of MCT oil (15 g 3 times daily) on a regular basis, and his psychiatric symptoms were stabilized. The hepatic hypovascular nodules have not increased in the 30 months since the initial EOB-MRI examination, and the patient remains under observation.
Figure 2.
Trends in plasma ammonia levels after taking MCT oil.
Discussion
Adult-onset type II citrullinemia (CTLN2) is a congenital metabolic disorder caused by an abnormality in citrin encoded by the SLC25A13 gene on the long arm of chromosome 7, and it is the adult form of citrin deficiency (3). Citrin serves as an aspartate-glutamate carrier (AGC) that transports aspartate from the mitochondria to the cytosol in exchange for glutamate in the cytosol (4). Abnormal citrin levels result in dysfunction of the argininosuccinate synthase (ASS) protein, which is a component of the urea cycle, and abnormal metabolism of ammonia. The symptoms include frequent loss of consciousness, convulsive seizures, personality change, and abnormal behavior. The patient had been stable without psychiatric symptoms since he was referred to our hospital for a while, but his levels of citrulline and ammonia in plasma were high. Thereafter, his psychiatric symptoms flared up again.
There are three pathological types of citrin deficiency: 1) neonatal biliary stasis due to citrin deficiency (NICCD), 2) stunted growth due to citrin deficiency, and 3) CTLN2. Neonatal cholestasis due to citrin deficiency is characterized by low birth weight and intrahepatic biliary stasis at one to four months old. In approximately 40% of cases, newborn mass screening reveals elevated levels of galactose, methionine, phenylalanine, and other amino acids (5). Most cases of NICCD are mild at approximately six months old and are cured by one year old. After NICCD is cured, the child develops a peculiar food preference for legumes and meat but not for carbohydrates, such as rice. In addition, some patients have growth retardation, which is termed “Failure to Thrive and Dyslipidemia Due to Citrin Deficiency” (FTTDCD) (6), and CTLN2 causes hyperammonemia-induced disturbances of consciousness. The present case showed neonatal jaundice and had a peculiar food preference since childhood. Thus, in retrospect, he was considered to have presented with the third pathological type of citrin deficiency.
The reason for the specific food preferences described above is that the glycolytic system does not function adequately in cases of citrin deficiency or dysfunction, which is responsible for transporting the intracellular NADH reduction equivalent generated by glucose metabolism into the mitochondria. Therefore, patients with citrin deficiency avoid the intake of carbohydrates that raise intracellular NADH and prefer to consume soy products rich in aspartic acid, which is a substrate for ASS (7). In the present case, the patient was also unable to consume carbohydrates, such as rice and udon noodles, and showed a peculiar dietary habit of preferring fried chicken, fish, cheese, squid, etc., foods that are rich in aspartic acid. It is important to confirm the patient's eating habits by a detailed inquiry, as in this case, to make a definite diagnosis. The four most common triggers for the development of CTLN2 are stress (overwork, dietary changes due to travel, etc.), an impaired liver function, alcohol consumption, and drugs (8). In retrospect, our patient had developed CTLN2 due to his restricted dietary intake during imprisonment. Thus, the novelty of this case, which separates it from previous reports, is that he developed CTLN2 in the special environment of imprisonment, where dietary intake was restricted and aversive reactions to the diet were difficult to elicit.
A low-protein, high-sugar diet, a common treatment for hepatic encephalopathy in CTLN2, exacerbates hyperammonemia (9). Glyceol, which is commonly used to prevent cerebral edema, is contraindicated because its metabolites increase intracellular NADH and may exacerbate cerebral edema (10). Diabetes mellitus is the other exacerbating factor of the syndrome, and treatment of diabetes mellitus is considered essential (11). In addition, there have been reports of cases of hyperlipidemia worsened by the administration of fibrate drugs, and it is possible that they may inhibit the effect of MCT oil, as discussed below, so they should not be administered (11,12). Treatment includes a low-carbohydrate, high-protein, high-lipid diet, but the only established treatment is liver transplantation, and patients who undergo liver transplantation are reported to have a good prognosis (13). However, although liver transplantation is effective, it is associated with problems of organ donation, surgical risks, and susceptibility to infection due to the use of immunosuppressive drugs. In recent years, MCT oil has been considered an effective treatment for CTLN2 because it provides energy to hepatocytes, improves the NADH/NAD+ ratio in the cells, improves impaired consciousness, and lowers citrulline levels (14). In the present case, the intake of MCT oil was started when CTLN2 was strongly suspected. After the encephalopathy flared up, a fixed amount of MCT oil was taken on a regular basis, and the patient's psychiatric symptoms did not worsen. In addition, administration of mRNA encoding human citrin has been reported as a treatment for citrin deficiency in animal experiments and is expected to be established as a treatment method for humans in the future (15).
CTLN2 can be associated with HCC, which is characterized by a younger age of onset and lack of cirrhosis than HCC with a background of viral hepatitis (2). The present patient has hypovascular nodules at present, and we diagnosed them as regenerating or dysplastic nodules. Careful imaging follow-up is required, as the 2- and 3-year cumulative incidence of hypovascularized nodules in the hepatocellular phase of EOB-MRI progressing to hypervascularization and diagnosed as HCC has been reported to be 11% and 16%, respectively (16).
In conclusion, young patients with liver damage of unknown cause should be carefully interviewed for a specific dietary history and examined with CTLN2 in mind.
Author's disclosure of potential Conflicts of Interest (COI).
Hiromi Kataoka: Lecture fees, Takeda Pharmaceutical and Otsuka Pharmaceutical.
References
- 1.Yamasaki M, Shimada T, Hamaoka S, Shibata M, Naito Y. [A case of adult-onset type II citrullinemia (CTLN2) triggered by an overseas travel]. Rinsho Shinkeigaku 54: 747-750, 2014. (in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 2.Nakayama M, Okamoto Y, Morita T, et al. Promoting effect of citrulline in hepatocarcinogenesis: possible mechanism in hypercitrullinemia. Hepatology 11: 819-823, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi K, Sinasac DS, Iijima M, et al. The gene mutated in adult-onset type II citrullinaemia encodes a putative mitochondrial carrier protein. Nat Genet 22: 159-163, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Saheki T, Kobayashi K. Mitochondrial aspartate glutamate carrier (citrin) deficiency as the cause of adult-onset type II citrullinemia (CTLN2) and idiopathic neonatal hepatitis (NICCD). J Hum Genet 47: 333-341, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Ohura T, Kobayashi K, Tazawa Y, et al. Neonatal presentation of adult-onset type II citrullinemia. Hum Genet 108: 87-90, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Numakura C, Tamiya G, Ueki M, et al. Growth impairment in individuals with citrin deficiency. J Inherit Metab Dis 42: 501-508, 2019. [DOI] [PubMed] [Google Scholar]
- 7.Yazaki M, Ikeda S, Kobayashi K, Saheki T. [Therapeutic approaches for patients with adult-onset type II citrullinemia (CTLN2): effectiveness of treatment with low-carbohydrate diet and sodium pyruvate]. Rinsho Shinkeigaku 50: 844-847, 2010. (in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 8.Shiohama N, Sugita Y, Imamura N, Sato T, Mizuno Y. [Type II citrullinemia triggered by acetaminophen]. No To Shinkei 45: 865-870, 1993. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 9.Imamura Y, Kobayashi K, Shibatou T, et al. Effectiveness of carbohydrate-restricted diet and arginine granules therapy for adult-onset type II citrullinemia: a case report of siblings showing homozygous SLC25A13 mutation with and without the disease. Hepatol Res 26: 68-72, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Yazaki M, Takei Y, Kobayashi K, Saheki T, Ikeda S. Risk of worsened encephalopathy after intravenous glycerol therapy in patients with adult-onset type II citrullinemia (CTLN2). Intern Med 44: 188-195, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe Y, Numakura C, Tahara T, et al. Diabetes mellitus exacerbates citrin deficiency via glucose toxicity. Diabetes Res Clin Pract 164: 108159, 2020. [DOI] [PubMed] [Google Scholar]
- 12.Hayasaka K, Numakura C, Yamakawa M, et al. Medium-chain triglycerides supplement therapy with a low-carbohydrate formula can supply energy and enhance ammonia detoxification in the hepatocytes of patients with adult-onset type II citrullinemia. J Inherit Metab Dis 41: 777-784, 2018. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda S, Yazaki M, Takei Y, et al. Type II (adult onset) citrullinaemia: clinical pictures and the therapeutic effect of liver transplantation. J Neurol Neurosurg Psychiatry 71: 663-670, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayasaka K, Numakura C, Toyota K, et al. Medium-chain triglyceride supplementation under a low-carbohydrate formula is a promising therapy for adult-onset type II citrullinemia. Mol Genet Metab Rep 1: 42-50, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao J, An D, Galduroz M, et al. mRNA therapy improves metabolic and behavioral abnormalities in a murine model of citrin deficiency. Mol Ther 27: 1242-1251, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akai H, Matsuda I, Kiryu S, et al. Fate of hypointense lesions on Gd-EOB-DTPA-enhanced magnetic resonance imaging. Eur J Radiol 81: 2973-2977, 2012. [DOI] [PubMed] [Google Scholar]