Abstract
A 55-year-old patient was admitted for variceal treatment, a complication of chronic portal hypertension and liver cirrhosis. Imaging studies revealed prominent duodenal varices, the pancreaticoduodenal vein as its afferent pathway, a drainer vessel into the inferior vena cava, and a paraumbilical vein. We successfully performed complete obliteration of the varix, including its afferent and efferent vessels, via the paraumbilical vein approach.
Keywords: liver cirrhosis, duodenal varices, portal hypertension, paraumbilical vein approach
Introduction
Duodenal varices (DV) are an ectopic manifestation of portal hypertension and liver cirrhosis. They develop in the submucosal and serosal layers of the duodenum. While not the most frequent complication of portal hypertension, bleeding from DV can be fatal (1).
We herein report the first case of endovascular treatment of DV accessed via the paraumbilical vein.
Case Report
A 55-year-old woman was referred to our hospital due to DV detected during a computed tomography (CT) examination. The varices developed in the course of alcoholic liver disease with cirrhosis (Child-Pugh score 5 class A) and portal hypertension. Laboratory tests revealed irregularities in liver and pancreatic enzymes, ammonia, platelets, and albumin levels (Table). DV were diagnosed endoscopically in the ascending part of the duodenum. Their form was classified as moderately enlarged, beady varices (F2), according to the 2nd edition of the Japanese Research Society for Portal Hypertension system (2). There were no mucosal erosions or red color signs. However, an increase in size was noticed. The patient's medical history included esophageal varices treated with endoscopic injection sclerotherapy eight years prior. Contrast-enhanced CT confirmed multiple enlarged vessels in the duodenum and revealed a paraumbilical vein. Three-dimensional renders were constructed (Fig. 1).
Table.
Laboratory Data on Admission.
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| ALT: alanine aminotransferase, AMY: amylase, APTT: activated partial thromboplastin time, AST: aspartate aminotransferase, BUN: blood urea nitrogen, Cl: chloride, CRP: C-reactive protein, D-bil: direct bilirubin, F: female, GGT: gamma-glutamyl transferase, INR: international normalized ratio, K: potassium, LDH: lactate dehydrogenase, Na: sodium, NH3: ammonia, PT: prothrombin time, T-bil: total bilirubin | |||
| White blood cells (×103/μL) | 5.6 (3.3-8.6) | Total protein (g/dL) | 7.4 (6.6-8.1) |
| Red blood cells (×106/μL) | 4.49 (3.8-4.9) | Albumin (g/dL) | 3.2 (4.1-5.1) |
| Hemoglobin (g/dL) | 12.5 (11.6-14.8) | BUN (mg/dL) | 10 (8-20) |
| Platelets (×104/μL) | 8.4 (15.8-34.8) | Creatinine (mg/dL) | 0.74 (0.46-0.79) |
| CRP (mg/dL) | 0.11 (0.0-0.15) | Na (mEq/L) | 139 (138-145) |
| T-Bil (mg/dL) | 1.2 (0.4-1.5) | K (mEq/L) | 4.0 (3.6-4.8) |
| D-Bil (mg/dL) | 0.7 (0.0-0.4) | Cl (mEq/L) | 110 (101-108) |
| AST (U/L) | 76 (13-30) | NH3(μg/dL) | 98 (12-66) |
| ALT (U/L) | 42 (7-23) | PT (s) | 13.3 (10.5-14.0) |
| AMY (U/L) | 156 (44-132) | PT (%) | 73 (80-120) |
| GGT (U/L) | 289 (F: 9-32) | PT-INR | 1.16 (0.9-1.10) |
| LDH (U/L) | 192 (124-220) | APTT (s) | 27.6 (24-39) |
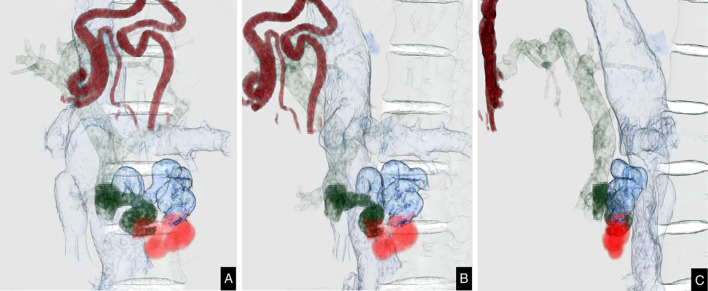
Figure 1.
3D projections constructed from preprocedural contrast-enhanced computed tomography (CT) images. Afferent vessel (pancreaticoduodenal vein): dark green, duodenal varices: orange, efferent vessel: blue, inferior vena cava: pale blue, paraumbilical vein: dark red, portal vein: pale green. A: coronal view. B: left anterior oblique view. C: left lateral view.
Due to the size of the DV and their tendency to grow, we suggested preventive treatment, and the patient was informed about possible options, including balloon-occluded retrograde transvenous obliteration, percutaneous transhepatic obliteration, percutaneous transparaumbilical sclerotherapy, and transjugular intrahepatic portosystemic shunt. On imaging, the retrograde pathway was challenging due to efferent vessel meandering and length, with the antegrade approach showing more promise, as a subcutaneous vein continuous with the paraumbilical vein was dilated enough to become a possible access route. We therefore opted to perform transparaumbilical sclerotherapy.
Ultrasound was used to confirm the location of the subcutaneous vein and assist with the initial puncture using a 20-G needle (Hakko Elaster Type-F IV Catheter; Hakko, Chikuma, Japan). A 4-Fr catheter (Impress Diagnostic Peripheral Catheter 4Fr; Merit Medical Japan, Tokyo, Japan) was advanced through the paraumbilical vein and into the portal vein (Fig. 2A-D). We then inserted a co-axial microcatheter system (Carnelian HF 2.6 Fr/2.8 Fr+Marvel Non Taper 1.9 Fr; Tokai Medical Products, Kasugai, Japan) into the feeding pancreaticoduodenal vein and then further into the varix (Fig. 2E). From within the varix, we obliterated the draining vein using 11 metal microcoils (Tornado Embolization Coil and Nester Embolization Coil; Cook Medical Japan, Tokyo, Japan), followed by 0.5 mL of N-butyl-2-cyanoacrylate (NBCA; Histoacryl; B. Braun Melsungen, Melsungen, Germany) mixed with ethiodized oil in a 1:4 ratio.
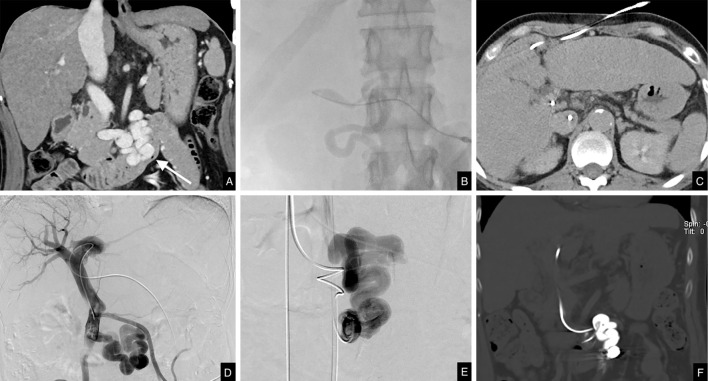
Figure 2.
Procedural images. A: Contrast-enhanced CT coronal portal phase image of the duodenal varices in the ascending part (arrow). B: Angiography during paraumbilical vein puncture. C: Percutaneous catheterization of the paraumbilical vein on axial CT. D: Percutaneous portography. E: Digital subtraction angiography of the varices. F: CT coronal image following embolization.
Once total obliteration of the efferent vessel had been confirmed, we proceeded to treat the varix and its afferent by applying 7 mL of 5% ethanolamine oleate in an iopamidol mixture (EOI) made by combining equal amounts of 10% ethanolamine oleate (Oldamin; ASKA Pharmaceutical, Tokyo, Japan) with 300 mgI/mL iopamidol (Iopamidol; Hikari Pharmaceuticals, Tokyo, Japan), containing gelatin sponge particles cut into 1- to 5-mm cubes (Fig. 2F).
Complete embolization of DV and their afferent and efferent vessels was confirmed on postprocedural contrast-enhanced CT. A pre- and postprocedural comparison can be seen on endoscopy and CT images (Fig. 3A, B, 3C, D, respectively).
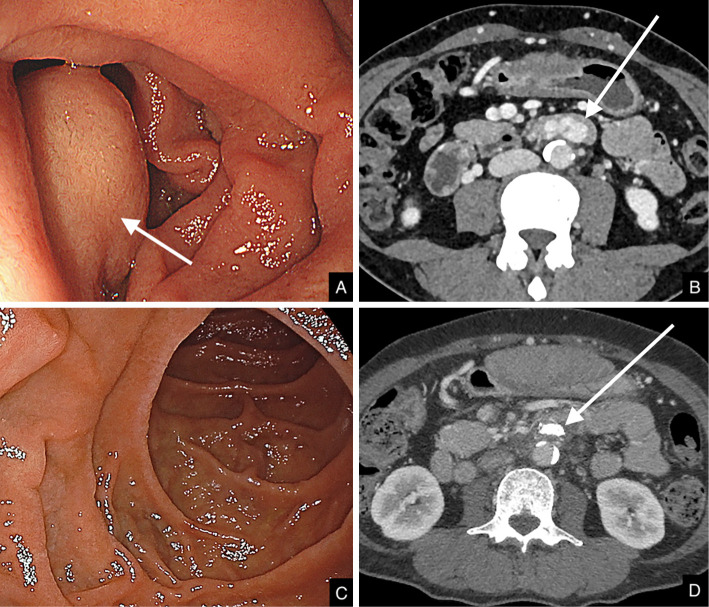
Figure 3.
Endoscopy and axial CT images before and after the procedure. A: Preprocedural endoscopy, varix protruding into the lumen (arrow). B: Preprocedural CT, visible varices in the ascending part of the duodenum (arrow). C: Postprocedural endoscopy, no visible structures protruding into the lumen. D: Postprocedural CT, varices disappeared, and NBCA cast residual seen as a high-density area (arrow).
Discussion
Portosystemic varices are dilated venous collateral vessels that develop in the course of portal hypertension. Gastroesophageal varices are most common, but ectopic instances are also encountered (3). Although DV account for 17% of all ectopic varices, their diagnosis rate on endoscopic examinations is reported to be 0.4% (1), which is thought to be due to their submucosal and serosal location (4). Bleeding from DV accounts for 1-5% of all variceal bleeds (5) but up to 33% of ectopic bleeds (3), with a high mortality rate of up to 40% (3,6). In our patient, the varices showed a progressive increase in size. Even though red color signs or erosion were not observed, some reports indicate that the risk factor for bleeding DV is their size (form), rather than red color signs (7). Endoscopic procedures are considered the first choice in variceal bleeding, but interventional radiology procedures are also frequently chosen in preventive cases, such as ours (8).
A dilated paraumbilical vein was observed in our patient on contrast-enhanced CT. These vessels develop in 19.3-20.2% of patients with chronic portal hypertension and liver cirrhosis (9,10). Paraumbilical vein puncture has been reported in portal vein targeting during transjugular intrahepatic portosystemic shunt procedure (10) as well as in varices at other locations. Paraumbilical vein puncture has been shown to be safe while also demonstrating a high success rate (10), and our case is the first one to have DV successfully managed using this approach. We were able to insert the catheter into the varix itself and into the vicinity of the efferent vein, from which it was possible to embolize it completely with NBCA and coils, followed by variceal obliteration with 5% EOI. This outflow embolization method is similar to how gastric varices are treated during balloon-occluded retrograde transvenous obliteration. Recent developments in catheter-based treatments have enabled operators to advance microcatheters into the varices, thus enabling better (and often complete) occlusion of the efferent vessels. Transparaumbilical sclerotherapy is a valid treatment option for patients with paraumbilical veins when retrograde transvenous or transhepatic/splenic approaches are considered difficult or pose risks in cases with ruptured varices or significant ascites.
Endoscopy excels at visualizing culprit lesions and offers treatment options by means of variceal ligation or sclerotherapy. These techniques are also usually the first-choice treatments for variceal bleeding. Duodenal varices may be more challenging to treat endoscopically than esophageal varices due to their location in the outer layers of the duodenal wall (4). They may also be difficult to reach if located in the ascending part of the duodenum. Among interventional radiology procedures, balloon-occluded retrograde transvenous obliteration has gained recognition in treating gastric varices, and its successful use in DV treatment has also been demonstrated (11). In our case, the retrograde pathway was more challenging due to efferent vessel meandering and length. Transjugular intrahepatic portosystemic shunt is another technique that has been used to treat DV (12). It alleviates portal hypertension and is considered safe and effective in cases of gastrointestinal hemorrhaging. Previous reports have shown mixed results regarding postprocedural control of DV rebleeding. Finally, successful percutaneous transhepatic or transsplenic approaches have also been reported (13). There is a potential risk of complications related to the direct puncture of the abdominal organs, such as biliary or intra-abdominal bleeding, and the procedure is contraindicated in patients with ascites. In our case, the dilated subcutaneous and paraumbilical veins were easily punctured and accessed and thus posed relatively little risk. Surgery is performed when the above approaches are contraindicated or may not provide satisfactory results.
Conclusion
The paraumbilical vein approach was useful for accessing and treating DV, and outflow embolization was useful for ensuring the effectiveness of sclerotherapy. It is a valid treatment option for patients with paraumbilical veins in whom retrograde transvenous or transhepatic/splenic approaches are challenging or contraindicated.
Informed consent was obtained from all individual participants included in this study.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Hashizume M, Tanoue K, Ohta M, et al. Vascular anatomy of duodenal varices: angiographic and histopathological assessments. Am J Gastroenterol 88: 1942-1945, 1993. [PubMed] [Google Scholar]
- 2.Tajiri T, Yoshida H, Obara K, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition): endoscopy of esophagogastric varices. Dig Endosc 22: 1-9, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Saad WEA, Lippert A, Saad NE, Caldwell S. Ectopic varices: anatomical classification, hemodynamic classification, and hemodynamic-based management. Tech Vasc Interv Radiol 16: 108-125, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Attila T, Kolbeck KJ, Bland ZM, Wang A, Rodriguez SA. Duodenal variceal bleeding successfully treated with transjugular intrahepatic portosystemic shunt: a case report and review of the literature. Turk J Gastroenterol 19: 284-290, 2008. [PubMed] [Google Scholar]
- 5.Norton ID, Andrews JC, Kamath PS. Management of ectopic varices. Hepatology 28: 1154-1158, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Khouqeer F, Morrow C, Jordan P. Duodenal varices as a cause of massive upper gastrointestinal bleeding. Surgery 102: 548-552, 1987. [PubMed] [Google Scholar]
- 7.Matsui S, Kudo M, Ichikawa T, Okada M, Miyabe Y. The clinical characteristics, endoscopic treatment, and prognosis for patients presenting with duodenal varices. Hepatogastroenterology 55: 959-962, 2008. [PubMed] [Google Scholar]
- 8.Watanabe N, Toyonaga A, Kojima S, et al. Current status of ectopic varices in Japan: results of a survey by the Japan Society for Portal Hypertension. Hepatol Res 40: 763-776, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Shi Q, Xiong K, Ding B, Ye X. Clinical characteristics of cirrhosis patients with umbilical vein recanalization: a retrospective analysis. Medicine (Baltimore) 100: e26774, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chin MS, Stavas JM, Burke CT, Dixon RG, Mauro MA. Direct puncture of the recanalized paraumbilical vein for portal vein targeting during transjugular intrahepatic portosystemic shunt procedures: assessment of technical success and safety. J Vasc Interv Radiol 21: 671-676, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka O, Ohno K, Ohno T, et al. Should balloon-occluded retrograde transvenous obliteration be the first-line interventional radiologic treatment for bleeding duodenal varices? A case report and review of the literature. Acta Radiol 49: 32-36, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Lopera JE, Arthurs B, Scheuerman C, Sandoz C, Petersosn S, Castaneda-Zuniga W. Bleeding duodenal: varices treatment by TIPS and transcatheter embolization. Cardiovasc Intervent Radiol 31: 431-434, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Kim TH, Choi JW, et al. A case report of bleeding from duodenal varices treated with percutaneous transhepatic obliteration. Int J Gastrointest Interv 8: 174-177, 2019. [Google Scholar]