Abstract
Background
Limited and conflicting data exist regarding the impact of first-trimester nursing occupational exposures on hypertensive disorders of pregnancy (HDP).
Aims
To investigate whether first-trimester night shift work, work hours and work-related activities are associated with HDP.
Methods
We conducted a cross-sectional analysis of 6610 women within the Nurses’ Health Study II. We used multiple logistic regression to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for the relation of occupational exposures to HDP.
Results
Nine per cent of respondents reported an HDP in the index pregnancy (gestational hypertension: n = 354, 5%, preeclampsia: n = 222, 3%). First-trimester fixed or rotating night shift work was not significantly associated with gestational hypertension or preeclampsia compared to day shift work only. Compared to those working 21–40 h/week, working overtime (≥41 h/week) was not associated with gestational hypertension but was associated with 43% higher odds of preeclampsia (95% CI 1.02, 2.00). For part-time work (≤20 h/week), the OR was 0.76 (95% CI 0.56, 1.02) for gestational hypertension and 0.64 (95% CI 0.43, 0.97) for preeclampsia. The odds of preeclampsia were 3% higher per additional hour worked per week (95% CI 1.01–1.04). Compared to 0–4 h spent standing or walking per day, standing or walking ≥9 h daily was associated with 32% lower odds of gestational hypertension (95% CI 0.47, 0.99) but was not significantly associated with preeclampsia. Frequency of heavy lifting was not associated with either hypertensive disorder or pregnancy.
Conclusions
Among nurses, working overtime was associated with higher odds of preeclampsia.
Nurses work in a unique occupational environment that can require night shift work, long working hours, prolonged standing and heavy lifting. In this study of US nurses (n = 6610), we observed an association between higher first-trimester working hours and higher odds of both preeclampsia and gestational hypertension. Our findings suggest that consideration of optional work-hour limitations during pregnancy could be beneficial.
Key learning points.
What is already known about this subject:
Limited and conflicting data exist regarding the impact of first-trimester nursing occupational exposures (including night shift work, work hours and work-related activities) on hypertensive disorders of pregnancy.
What this study adds:
Night shift work during the first trimester was not associated with HDP.
Working overtime was associated with higher odds of preeclampsia and working part time was associated with lower odds of both gestational hypertension and preeclampsia.
The odds of preeclampsia were 3% higher per additional hours worked per week.
What impact this may have on practice or policy:
Although a causal relationship between work hours and HDP has not been established and the underlying biological mechanisms are not yet understood, our work suggests that consideration of optional work-hour limitations during pregnancy may have beneficial effects.
Future research is needed to confirm the association between work hours and HDP and future interventional trials of modified work schedules could be considered.
Introduction
Over 4.9 million nurses are employed in the USA, and approximately half of them are women of reproductive age [1]. Nurses work in a unique occupational environment that can require rotating work schedules, night shifts, long hours, prolonged standing and heavy lifting. Recent studies found that night shift work and long working hours may be associated with an increased risk of adverse pregnancy outcomes [2], including spontaneous abortion [3] and preterm delivery [4]. Potential physiological mechanisms include disruption of normal circadian behavioural and physiologic pathways, shortened sleep duration, and neuroendocrine dysregulation [5].
Hypertensive disorders of pregnancy (HDP), including preeclampsia and gestational hypertension, together affect 18 million women globally and are a leading cause of maternal and foetal morbidity and mortality [6]. These disorders are typically diagnosed after 20 weeks gestational age. The known risk factors include advanced maternal age, primiparity, multifetal pregnancy, alcohol use, and overweight and obesity [7]. The prevalence of HDP has increased since the start of the COVID-19 pandemic [8], possibly owing to greater rates of home quarantine and decreased physical activity. HDP are relatively understudied with respect to occupational exposures compared to other pregnancy outcomes [2]. The few studies that have been conducted on occupational exposures and HDP have demonstrated conflicting results [9–13]. Thus, we investigated the association between first-trimester nursing occupational exposures (including night shift work, work hours and work-related activities) and HDP.
Methods
The Nurses’ Health Study II (NHS II) is a US cohort of 116 429 female nurses aged 25–42 at enrolment in 1989. Participants are followed via questionnaires mailed every 2 years. On the 2001 follow-up questionnaire, participants were asked if they (i) had a pregnancy since 1993; (ii) worked as a nurse during the most recent pregnancy; and (iii) would be willing to complete a supplemental questionnaire concerning occupational exposures during the most recent (index) pregnancy. An occupational supplement was mailed to women who answered yes to all three questions. The supplement included detailed questions about occupational exposures during the index pregnancy, including night shift work, work hours and work-related activities.
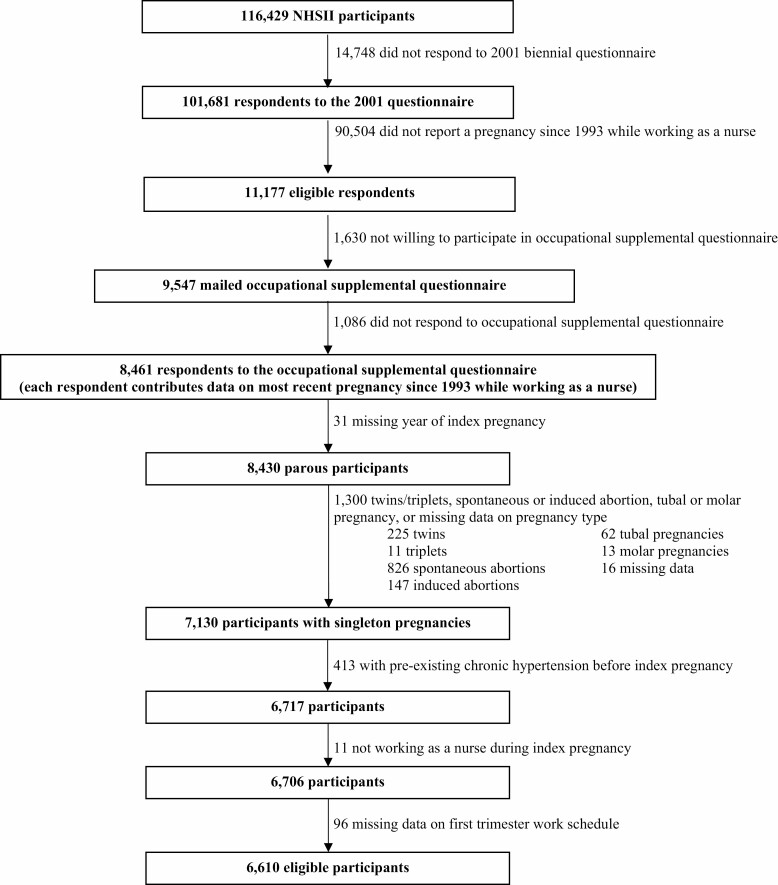
Of 101 681 respondents to the main 2001 questionnaire, 11 177 (11%) indicated that they had experienced a pregnancy since 1993. Of these women, 9547 (85%) indicated willingness to respond to a supplemental questionnaire. Of the 9547 women who were mailed the supplemental questionnaire, 8461 responses were received (89%). We excluded 1300 pregnancies in total, including those resulting in twins (n = 225), triplets (n = 11), spontaneous abortion (n = 826), induced abortion (n = 147), tubal pregnancy (n = 62), molar pregnancy (n = 13) or those with missing data on pregnancy outcomes (n = 16). We further excluded pregnancies with missing data on the year of index pregnancy (n = 31) or first-trimester work exposures (n = 96). Pregnancies were excluded if participants reported pre-existing chronic hypertension before index pregnancy (n = 413) or if participants were not working as a nurse during the index pregnancy (n = 11). This left 6610 pregnancies for analysis (Figure 1).
Figure 1.
Nurses’ Health Study II participant eligibility flowchart.
The NHS II occupational supplement assessed first-trimester work schedule (days only, evenings only, nights only, rotating with nights, rotating without nights, other/didn’t work), average hours worked per week (none, 1–20, 21–40, 41–60, ≥61 h/week), hours per day of standing or walking at work (<1, 1–4, 5–8, ≥9 h/day), and how often during the average day the respondent lifted 25 pounds or more at work (never, 1–5, 6–15, 16–30, ≥31 times/day). Night shift was defined as most of the work hours falling between midnight and 8 a.m. We chose to analyse occupational exposures from the first trimester instead of the second or third trimester to ensure exposures were measured before the development of clinical outcomes and to avoid bias due to reverse causation.
Participants self-reported whether they had been diagnosed with gestational hypertension and/or preeclampsia on the NHS II occupational supplement. Participants who reported a history of both gestational hypertension and preeclampsia were classified as having had preeclampsia only and were not additionally counted as cases of gestational hypertension. Self-reports of preeclampsia have previously been validated against medical records in a subgroup of 598 NHS II participants [14], with a positive predictive value of 89% when compared against medical records with sufficient information to establish a diagnosis [15].
The NHS II occupational supplement assessed history of gestational hypertension or preeclampsia (before the index pregnancy), work setting, and first-trimester smoking, coffee, caffeinated soda or tea, and alcohol consumption. Work setting was defined as acute care: critical care, emergency room, operating room; non-acute care: medical/surgical floor, general paediatrics, rehabilitation, home/community, education/research, administration; or other/missing. The main biennial NHS II questionnaire assessed maternal age, race/ethnicity, geographical region, pre-pregnancy body mass index (BMI), pre-pregnancy physical activity, pre-pregnancy neighbourhood socio-economic status (SES) score, husband’s level of education and parity.
Age-adjusted means and proportions of selected characteristics were calculated. We used multiple logistic regression to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for the association of occupational exposures with gestational hypertension and preeclampsia. Occupational exposures were grouped as follows: work schedule: fixed or rotating nights, rotating shifts in evenings without nights and days only; hours worked per week: 1–20, 21–40 and ≥41 h/week; hours spent standing or walking per day at work: 0–4, 5–8 and ≥9 h/day; number of times engaged in heavy lifting per day at work: none, 1–5 and ≥6 times/day. Multivariable-adjusted models included the following covariates: age (years), race/ethnicity (Hispanic or non-White, non-Hispanic White), region (Northeast, Midwest, South, West), work setting (acute care, non-acute care, other/missing), pre-pregnancy BMI (kg/m2), pre-pregnancy physical activity (< 9, 9–26, ≥27 MET-h/week), pre-pregnancy neighbourhood SES (continuous), husband’s level of education (high school or less, some or all college, graduate school, other/missing), parity (yes/no), history of gestational hypertension (yes/no), history of preeclampsia (yes/no), and first-trimester smoking (yes/no), coffee (<1 cup per week, 1 cup per week—1 cup per day, ≥2 cups per day), caffeinated soda or tea (<1 serving per week, 1 serving per week—1 serving per day, ≥2 servings per day), and alcohol intake (none, <1 drink per week, ≥1 drinks per week). Multivariable-adjusted models included mutual adjustment for all occupational factors. To calculate P for trends for specific occupational exposures, the midpoint of each reporting category was used to create continuous variables. As a sensitivity analysis, we investigated whether relationships between occupational exposures and HDP were preserved among women who drank<1 drink per week and did not smoke. The study was approved by the Institutional Review Board of the Brigham and Women’s Hospital.
Results
Among 6610 confirmed pregnancies during which the mother reported working as a nurse in the first trimester, 354 (5%) resulted in gestational hypertension and 222 (3%) resulted in preeclampsia. Most women reported working a regular day schedule during their first trimester (68%), 17% reported working evenings or rotating between day and evening shifts, 9% reported working a fixed night schedule, and 6% reported working a rotating schedule that included nights. Overall, 17% of women reported working more than 40 h/week, on average, during their first trimester, and 26% reported working part time.
Table 1 shows the age-adjusted proportion of selected characteristics of the study population by category of shift work during the first trimester. Women who reported working fixed or rotating nights had, on average, a higher BMI and consumed more caffeinated beverages per day during the first trimester compared with day and evening workers. Women who reported working fixed or rotating nights were also more likely to work in an acute care setting than in a non-acute care setting compared with day and evening workers.
Table 1.
Age-standardized characteristics of the study population by first-trimester work schedule
| First-trimester work schedule | |||
|---|---|---|---|
| Days only (n = 4494, 68%) | Evenings or rotating shifts without nights (n = 1120, 17%) | Fixed or rotating nights (n = 996, 15%) |
|
| Maternal age at index pregnancy, mean (SD) | 36.6 (3.4) | 35.9 (3.3) | 35.9 (3.4) |
| Hispanic or non-White, % | 4 | 3 | 5 |
| Region, % | |||
| Northeast | 32 | 39 | 33 |
| Midwest | 35 | 37 | 36 |
| South | 19 | 12 | 17 |
| West | 14 | 11 | 14 |
| Pre-pregnancy BMI (kg/m2), % | |||
| <25 | 71 | 71 | 66 |
| 25–29 | 19 | 19 | 23 |
| ≥30 | 10 | 9 | 11 |
| Pre-pregnancy physical activity (MET-h/week), % | |||
| <9 | 36 | 35 | 37 |
| 9–26 | 35 | 36 | 34 |
| ≥27 | 29 | 29 | 29 |
| Pre-pregnancy SES scorea | 0.70 (4.61) | 0.97 (4.11) | 0.18 (4.67) |
| Husband’s level of education, % | |||
| High school or less | 15 | 15 | 18 |
| College (2 or 4 years) | 50 | 53 | 52 |
| Graduate school | 29 | 28 | 25 |
| Other/missing | 5 | 4 | 5 |
| First-trimester caffeinated coffee intake (1 cup = 8 oz), % | |||
| None or <1 cup/week | 63 | 62 | 60 |
| 1 cup/week–1 cup/day | 28 | 29 | 27 |
| ≥2 cups/day | 10 | 9 | 13 |
| First-trimester caffeinated soda or tea intake (1 serving = 12 oz soda, 8 oz tea or 16 oz iced tea), % | |||
| None or <1 serving/week | 49 | 50 | 43 |
| 1 serving/week–1 serving/day | 40 | 42 | 44 |
| ≥2 servings/day | 11 | 9 | 13 |
| First-trimester alcohol intake (1 drink = 12 oz beer, 6 oz wine, or 1 oz liquor), % | |||
| None | 81 | 82 | 84 |
| <1 drink/week | 14 | 14 | 13 |
| ≥1 drinks/week | 5 | 4 | 3 |
| First-trimester smoked cigarettes, % | 6 | 5 | 6 |
| Work setting,b % | |||
| Acute care setting | 24 | 36 | 48 |
| Non-acute care setting | 44 | 35 | 24 |
| Other/missing | 32 | 29 | 28 |
| Parous at index pregnancy, % | 84 | 90 | 88 |
| Prior gestational hypertension,c % | 11 | 11 | 10 |
| Prior preeclampsia,c % | 7 | 7 | 7 |
| Morning preference chronotype, % | 64 | 51 | 46 |
| First-trimester hours worked per week, % | |||
| 1–20 | 21 | 46 | 22 |
| 21–40 | 59 | 49 | 64 |
| ≥41 | 20 | 6 | 13 |
| First-trimester hours spent standing per day at work, % | |||
| 0–4 | 40 | 16 | 11 |
| 5–8 | 44 | 63 | 47 |
| ≥9 | 17 | 21 | 42 |
| First-trimester number of times heavy lifting per day at work,d % | |||
| None | 45 | 22 | 20 |
| 1–5 | 38 | 48 | 42 |
| ≥6 | 17 | 30 | 38 |
Values are means (SD) for continuous variables, percentages for categorical variables and are standardized to the age distribution of the study population (except maternal age at index pregnancy).
aSES is based on the sum of the z-scores of census tract indicators based on participants’ zip codes (median household income, home value, percentage with college degree, percentage of families with interest or dividends, percentage occupied housing, percentage living in poverty, percentage White).
bAcute care settings include critical care, emergency room, operating room; non-acute care settings include medical/surgical floor, general paediatrics, rehabilitation, home/community, education/research or administration.
cPer cent of patients calculated from among parous participants only (nulliparous women excluded).
dLifting refers to lifting or moving a physical load of 25 pounds or more, including repositioning or transferring patients.
Table 2 provides the estimated ORs for gestational hypertension for occupational exposures. Age-adjusted and multivariable-adjusted results were generally similar. In multivariable-adjusted results, first-trimester fixed or rotating night shift work was not significantly associated with gestational hypertension (OR = 1.30, 95% CI 0.95, 1.79) compared to day shift work only. Working overtime (>40 h/week) was not associated with gestational hypertension (OR = 1.02, 95% CI 0.76, 1.37) compared to working 21–40 h/week. Working part time (≤20 h/week) was inversely associated with gestational hypertension, though this finding was not statistically significant (OR = 0.76, 95% CI 0.56, 1.02). There was no statistically significant trend between higher work hours per week and odds of gestational hypertension (OR = 1.01/h worked, 95% CI 1.00, 1.02; P-trend = 0.07). Compared to 0–4 h spent standing or walking per day, standing or walking ≥9 h daily was associated with a statistically significant 32% lower odds of gestational hypertension (OR = 0.68, 95% CI 0.47, 0.99); this translated to a 5% reduction in odds of gestational hypertension per hour of standing or walking that was borderline significant (95% CI 0.91, 1.00, P-trend = 0.06). Frequency of heavy lifting was not associated with gestational hypertension (OR = 1.02 per time heavy lifting, 95% CI 0.96,1.08; P-trend = 0.54).
Table 2.
Odds ratios and 95% confidence intervals of gestational hypertension (n = 354 cases) by first-trimester occupational exposure in the NHS II (n = 6610 participants, 1993–2001)
| N cases/N exposed | Age-adjusteda | Multivariable-adjustedb | |||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| Work schedule | |||||
| Fixed or rotating nights | 65/996 | 1.27 | 0.95, 1.68 | 1.30 | 0.95, 1.79 |
| Evenings or rotating shifts without nights | 48/1120 | 0.81 | 0.59, 1.11 | 0.89 | 0.63, 1.26 |
| Days only (ref) | 241/4,494 | 1.00 | – | 1.00 | – |
| Hours worked per week | |||||
| 1–20 | 67/1697 | 0.68 | 0.51, 0.90 | 0.76 | 0.56, 1.02 |
| 21-40 (ref) | 218/3812 | 1.00 | – | 1.00 | – |
| ≥41 | 69/1101 | 1.09 | 0.82, 1.44 | 1.02 | 0.76, 1.37 |
| Per hour worked | 1.02 | 1.01, 1.03 | 1.01 | 1.00, 1.02 | |
| P-trend | 0.003 | 0.07 | |||
| Hours spent standing per day at work | |||||
| 0–4 (ref) | 122/2,076 | 1.00 | -- | 1.00 | -- |
| 5–8 | 167/3,145 | 0.92 | 0.72, 1.17 | 0.84 | 0.64, 1.12 |
| ≥9 | 65/1,389 | 0.82 | 0.60, 1.12 | 0.68 | 0.47, 0.99 |
| Per hour spent standing | 0.98 | 0.94, 1.02 | 0.95 | 0.91, 1.00 | |
| P-trend | 0.23 | 0.06 | |||
| Number of times engaged in heavy lifting per day at work | |||||
| None (ref) | 132/2482 | 1.00 | – | – | – |
| 1–5 | 136/2668 | 0.99 | 0.77, 1.26 | 1.03 | 0.78, 1.36 |
| ≥6 | 86/1460 | 1.17 | 0.88, 1.55 | 1.12 | 0.79, 1.60 |
| Per time heavy lifting | 1.02 | 0.98, 1.07 | 1.02 | 0.96, 1.08 | |
| P-trend | 0.34 | 0.54 | |||
aAdjusted for age only. These models test associations for each occupational factor separately.
bAdjusted for age (years), race/ethnicity (Hispanic or non-White, non-Hispanic White), region (Northeast, Midwest, South, West), work setting (acute care, non-acute care, other/missing), pre-pregnancy body mass index (kg/m2), pre-pregnancy physical activity (< 9, 9–26, ≥27 MET-h/week), pre-pregnancy neighbourhood socio-economic status score (continuous), husband’s level of education (high school or less, some or all college, graduate school, other/missing), parity (yes/no), history of gestational hypertension (yes/no), history of preeclampsia (yes/no), and first-trimester smoking (yes/no), coffee (<1 cup/week, 1 cup/week–1 cup/day, ≥2 cups/day), caffeinated soda or tea (<1 serving/week, 1 serving/week–1 serving/day, ≥2 servings/day), and alcohol intake (none, <1 drink/week, ≥1 drinks/week). This model tests associations for each occupational factor independently (with mutual adjustment in models).
Table 3 provides the estimated ORs for preeclampsia for occupational exposures. Age-adjusted and multivariable-adjusted results were generally similar. In multivariable-adjusted results, first-trimester fixed or rotating night shift work was not significantly associated with preeclampsia (OR = 0.81, 95% CI 0.53, 1.22) compared to day work only. Compared to those working 21–40 h/week, working overtime was associated with 43% higher odds of preeclampsia (95% CI 1.02, 2.00) and part-time work was associated with 36% lower odds of preeclampsia (95% CI 0.43, 0.97); for every hour worked, the odds of preeclampsia rose by 3% (OR = 1.03/h worked, 95% CI 1.01, 1.04; P-trend = 0.002). Hours spent standing or walking per day (P-trend = 0.60) and frequency of heavy lifting were not associated with preeclampsia (P-trend = 0.37).
Table 3.
Odds ratios and 95% confidence intervals of preeclampsia (n = 222 cases) by first-trimester occupational exposure in the NHS II (n = 6610 participants, 1993–2001)
| N cases/N exposed | Age-adjusteda | Multivariable-adjustedb | |||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| Work schedule | |||||
| Fixed or rotating nights | 33/996 | 0.97 | 0.66, 1.43 | 0.81 | 0.53, 1.22 |
| Evenings or rotating shifts without nights | 34/1120 | 0.89 | 0.61, 1.30 | 1.01 | 0.68, 1.52 |
| Days only (ref) | 155/4494 | 1.00 | -- | 1.00 | -- |
| Hours worked per week | |||||
| 1–20 | 32/1697 | 0.53 | 0.36, 0.79 | 0.64 | 0.43, 0.97 |
| 21–40 (ref) | 133/3,812 | 1.00 | -- | 1.00 | -- |
| ≥41 | 57/1,101 | 1.50 | 1.09, 2.06 | 1.43 | 1.02, 2.00 |
| Per hour worked | 1.04 | 1.02, 1.05 | 1.03 | 1.01, 1.04 | |
| P-trend | <0.0001 | 0.002 | |||
| Hours spent standing per day at work | |||||
| 0–4 (ref) | 63/2076 | – | – | 1.00 | – |
| 5–8 | 100/3145 | 1.07 | 0.77, 1.47 | 0.99 | 0.68, 1.42 |
| ≥9 | 59/1389 | 1.46 | 1.01, 2.10 | 1.17 | 0.75, 1.82 |
| Per hour spent standing | 1.05 | 0.99, 1.10 | 1.02 | 0.96, 1.08 | |
| P-trend | 0.09 | 0.60 | |||
| Number of times engaged in heavy lifting per day at work | |||||
| None (ref) | 70/2482 | 1.00 | – | 1.00 | – |
| 1-5 | 95/2668 | 1.30 | 0.95, 1.78 | 1.25 | 0.88, 1.78 |
| ≥6 | 57/1460 | 1.44 | 1.01, 2.06 | 1.21 | 0.78, 1.88 |
| Per time heavy lifting | 1.06 | 1.00, 1.13 | 1.03 | 0.96, 1.11 | |
| P-trend | 0.04 | 0.37 | |||
aAdjusted for age only. These models test associations for each occupational factor separately.
bSee footnote for Table 2.
A sensitivity analysis only among women who drank <1 drink per week and did not smoke (n = 5962) revealed similar results, with the difference that the trend between higher work hours per week and odds of gestational hypertension became statistically significant (Table 1, available as Supplementary data at Occupational Medicine Online).
Discussion
In this large cohort of US nurses, night shift work during the first trimester was not associated with HDP. However, working overtime was associated with higher odds of preeclampsia, and working part time was associated with lower odds of both gestational hypertension and preeclampsia. The odds of preeclampsia were 3% higher per additional hours worked per week. Other occupational exposures we examined were not associated with HDP, except for standing or walking≥9 h daily and gestational hypertension.
With respect to night shift work, our findings are consistent with three prior studies [10,11,16], which have all reported no association between night shift work and HDP. A separate study conducted in a primarily Latina population did find that shift work in general was associated with a higher prevalence of HDP; however, this study did not examine night shift work in relation to day work specifically [17]. One prior Danish study did find that among night shift workers, a higher number of consecutive night shifts was associated with a higher risk of preeclampsia [13]; however, this study did not find a difference in the risk of preeclampsia comparing night shift workers to day workers (which was our reference group). Importantly, the Danish study did not adjust for total work hours per week, which could be a potential confounder of the association between night shift work and HDP. Overall, existing evidence suggests that the way night shifts are organized, rather than mere participation in night shift work, maybe more relevant for the risk of HDP, but additional research is required to clarify this possibility.
Long working hours are common in nursing and have worsened since the COVID-19 pandemic due to critical staffing shortages. We observed a statistically significant trend between higher working hours and higher odds of both preeclampsia and gestational hypertension. Long working hours are theorized to have multiple downstream consequences that could impact pregnancy, including less time to sleep and recover from work, longer exposure to workplace hazards and demands, less time with family and less time for other leisure activities, including exercise [18]. These factors could lead to inadequate sleep, fatigue and stress, and may in turn increase unhealthy behaviours such as tobacco and caffeine use. Notably, our observed associations were robust after controlling for physical activity, smoking, alcohol use and caffeinated beverage consumption, and sensitivity analyses restricted to those with low alcohol use and never smokers did not significantly alter our findings. Additional research accounting for total sleep time, stress and social isolation is required to clarify potential mechanisms by which working hours may be linked to HDP.
Two previous studies did not observe a significant association between work hours and HDP [11,16], which is inconsistent with our findings. There are multiple possible explanations for this discrepancy, including that both previous studies (Generation R and the Taiwan Birth Cohort Study) were not conducted in the USA, and differences in healthcare systems, access to prenatal care and workplace policies could impact the relationship between work hours and HDP. Additionally, both previous studies included participants from a heterogeneous mix of occupations and industries, including manufacturing, construction, mining, retail, defence and others, while our study was limited to nurses. One of the previous studies [16] (Generation R) had rates of preeclampsia and gestational hypertension that were much lower than those observed in our cohort (1% versus 3% for preeclampsia; 2% versus 5% for gestational hypertension), which could have limited the power of the previous study to detect associations with HDP. Notably, the Generation R study defined HDP based on strict clinical criteria from medical record extraction, and the Taiwan Birth Cohort Study identified cases of HDP from birth registration, whereas our outcomes were ascertained by self-report. However, self-reports of preeclampsia have previously been validated in NHS II, with a positive predictive value of 89% when compared against medical records [15]. Differences in the frequency of HDP between our study and prior studies may be due to underlying differences in the populations of the cohorts. For example, participants in our cohort were generally older and parous at the time of the index pregnancy compared to the previous studies. However, a sensitivity analysis restricting to nulliparous participants in our study did yield similar results (though no longer statistically significant, likely due to far fewer cases). Other factors that could contribute to the discrepancy between our findings and the results from previous studies include differences in adjustment for confounding, exposure assessment, and prospective versus retrospective data collection.
Although prior studies have not demonstrated an association between work hours and HDP, a recent meta-analysis found that working more than 40 h/week was associated with multiple other adverse pregnancy outcomes that can be downstream consequences of HDP, including preterm birth, low birthweight, small for gestational age and spontaneous abortion [2]. Furthermore, a standing posture during the third trimester has been found to result in compressed pelvic vessels and a reduction in venous blood flow, which could contribute to adverse pregnancy outcomes [19]. Thus, there is data to suggest a plausible relationship between work hours and adverse pregnancy outcomes.
Preeclampsia and gestational hypertension share some, but not all, risk factors [20]. Consistent with the literature, we found an association between working overtime and preeclampsia, but not gestational hypertension. Whether preeclampsia and gestational hypertension represent different diseases affecting similar organs, or different severities of the same disease remains a topic of debate [20].
The strengths of our study include its large sample size, detailed assessment of multiple occupational exposures as well as robust assessment of potential confounding variables. We were able to adjust for both individual characteristics (including parity, history of preeclampsia and/or gestational hypertension, pre-pregnancy BMI, physical activity, and first-trimester smoking, caffeine and alcohol consumption), as well as socio-demographic variables (geographic region, neighbourhood SES and husband’s level of education). Another strength of our study is that by examining a national sample of US nurses, socio-economic variability and differences in working conditions were minimized and the likelihood of working rotating or night shifts was increased.
Our study has several limitations. Because we relied on self-report, pregnancy-related exposures and outcomes may have been inadequately recalled. However, nurses are well-educated professionals who are presumably more sensitized to health events than the general population. Moreover, respondents reported events that occurred relatively recently (within the last 8 years). It is possible that there was a healthy worker effect in our study, in that women who had healthier pregnancies were more able to perform heavy lifting or prolonged standing throughout their pregnancies. However, because we analysed data on occupational exposures during the first trimester, bed rest or other restrictions resulting from HDP diagnosis later in pregnancy are less likely to result in reverse causation. Finally, our data on occupational exposures are average values by trimester, and therefore we lack the granularity to examine early versus late first-trimester occupational exposures.
Though we did not observe an association between night shift work and HDP, our work suggests an association between long working hours and HDP. In the Nurses’ Worklife and Health study [21], one-third of nurses worked more than 40 h/week, and 17% reported having to participate in mandatory overtime. Although a causal relationship between work hours and HDP has not been established and the underlying biological mechanisms are not yet understood, our work suggests that consideration of optional work-hour limitations during pregnancy may have beneficial effects. Future research is needed to confirm the association between work hours and HDP and future interventional trials of modified work schedules could be considered.
Supplementary Material
Contributor Information
I Agarwal, Maine Health Institute for Research, Maine Medical Center, Scarborough 04074, MN, USA.
S Wang, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA 02115, USA.
J Stuart, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA 02115, USA; Channing Division of Network Medicine, Brigham and Women’s Hospital, Boston, MA 02115, USA.
S Strohmaier, Department of Epidemiology, Center for Public Health, Medical University of Vienna, Vienna 1090, Austria.
E Schernhammer, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA 02115, USA; Channing Division of Network Medicine, Brigham and Women’s Hospital, Boston, MA 02115, USA; Department of Epidemiology, Center for Public Health, Medical University of Vienna, Vienna 1090, Austria.
J Rich-Edwards, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA 02115, USA; Channing Division of Network Medicine, Brigham and Women’s Hospital, Boston, MA 02115, USA.
J H Kang, Channing Division of Network Medicine, Brigham and Women’s Hospital, Boston, MA 02115, USA.
Funding
NHS2 II cohort infrastructure is funded by U01 CA176726. E.S. is supported by ERC-2021-ADG CLOCKrisk, Grant agreement No. 101053225.
Competing Interests
Outside of this work, J.H.K. has received research funding from Pfizer, Inc.
References
- 1. Smiley RA, Ruttinger C, Oliveira CM et al. The 2020 National Nursing Workforce Survey. J Nurs Regul 2021;12:S1–S96. [Google Scholar]
- 2. Cai C, Vandermeer B, Khurana R et al. The impact of occupational shift work and working hours during pregnancy on health outcomes: a systematic review and meta-analysis. Am J Obstet Gynecol 2019;221:563–576. [DOI] [PubMed] [Google Scholar]
- 3. Whelan EA, Lawson CC, Grajewski B, Hibert EN, Spiegelman D, Rich-Edwards JW. Work schedule during pregnancy and spontaneous abortion. Epidemiology 2007;18:350–355. [DOI] [PubMed] [Google Scholar]
- 4. Lawson CC, Whelan EA, Hibert EN, Grajewski B, Spiegelman D, Rich-Edwards JW. Occupational factors and risk of preterm birth in nurses. Am J Obstet Gynecol 2009;200:51.e1–51.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gamble KL, Resuehr D, Johnson CH. Shift work and circadian dysregulation of reproduction. Front Endocrinol (Lausanne) 2013;4:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang W, Xie X, Yuan T et al. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: a population‐based study. BMC Pregnancy and Childbirth 2021;21:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang L, Tang K, Magee LA et al. A global view of hypertensive disorders and diabetes mellitus during pregnancy. Nat Rev Endocrinol 2022;18:760–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai QY, Yang Y, Wang YH et al. Home quarantine: a double-edged sword during COVID-19 pandemic for hypertensive disorders of pregnancy and the related complications. Diabetes Metab Syndr Obes 2022;15:2405–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nurminen T. Shift work, fetal development and course of pregnancy. Scandinavian J Work Environ Health 1989;15:395–403. [DOI] [PubMed] [Google Scholar]
- 10. Haelterman E, Marcoux S, Croteau A, Dramaix M. Population-based study on occupational risk factors for preeclampsia and gestational hypertension. Scand J Work Environ Health 2007;33:304–317. [DOI] [PubMed] [Google Scholar]
- 11. Chang PJ, Chu LC, Hsieh WS, Chuang YL, Lin SJ, Chen PC. Working hours and risk of gestational hypertension and pre-eclampsia. Occup Med (Lond) 2010;60:66–71. [DOI] [PubMed] [Google Scholar]
- 12. Wergeland E, Strand K. Working conditions and prevalence of pre-eclampsia, Norway 1989. Int J Gynaecol Obstet 1997;58:189–196. [DOI] [PubMed] [Google Scholar]
- 13. Hammer P, Flachs E, Specht I et al. Night work and hypertensive disorders of pregnancy: a national register-based cohort study. Scand J Work Environ Health 2018;44:403–413. [DOI] [PubMed] [Google Scholar]
- 14. Stuart JJ, Tanz LJ, Missmer SA et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Ann Intern Med 2018;169:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stuart JJ, Tanz LJ, Cook NR et al. Hypertensive disorders of pregnancy in 10-year cardiovascular disease risk prediction. J Am Coll Cardiol 2018;72:1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nugteren JJ, Snijder CA, Hofman A, Jaddoe VWV, Steegers EAP, Burdorf A. Work-related maternal risk factors and the risk of pregnancy induced hypertension and preeclampsia during pregnancy. The Generation R Study. PLoS One 2012;7:e39263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Larson JM, Bazalakova MH, Godecker A, DelBeccaro M, Aagaard KM, Antony KM. Shift work and sleep duration are associated with adverse pregnancy outcomes in a predominantly Latinx population with high rates of obesity. PLoS One 2022;17:e0272218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caruso CC. Possible broad impacts of long work hours. Ind Health 2006;44:531–536. [DOI] [PubMed] [Google Scholar]
- 19. Schneider KT, Huch A, Huch R. Premature contractions: are they caused by maternal standing?. Acta Genet Med Gemellol (Roma) 1985;34:175–178. [DOI] [PubMed] [Google Scholar]
- 20. Villar J, Carroli G, Wojdyla D et al.; World Health Organization Antenatal Care Trial Research Group. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstetr Gynecol 2006;194:921–931. [DOI] [PubMed] [Google Scholar]
- 21. Trinkoff A, Geiger-Brown J, Brady B, Lipscomb J, Muntaner C. How long and how much are nurses now working? Am J Nurs 2006;106:60–71, quiz 72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.