Abstract
Multisystemic inflammatory syndrome in adults is a hyperinflammatory condition following (within 4–12 weeks) SARS‐CoV‐2 infection. Here, the dysregulation of the immune system leads to a multiorgan involvement often affecting the heart. Cardiac involvement in multisystemic inflammatory syndrome in adults has been described mainly in young men without other comorbidities and may present with different clinical scenarios, including acute heart failure, life‐threatening arrhythmias, pericarditis, and myocarditis, with a nonnegligible risk of mortality (up to 7% of all cases). The heterogeneity of its clinical features and the absence of a clear case definition make the differential diagnosis with other postinfectious (eg, infective myocarditis) and hyperinflammatory diseases (eg, adult Still disease and macrophage activation syndrome) challenging. Moreover, the evidence on the efficacy of specific treatments targeting the hyperinflammatory response underlying this clinical condition (eg, glucocorticoids, immunoglobulins, and other immunomodulatory agents) is sparse and not supported by randomized clinical trials. In this review article, we aim to provide an overview of the clinical features and the diagnostic workup of multisystemic inflammatory syndrome in adults with cardiac involvement, highlighting the possible pathogenetic mechanisms and the therapeutic management, along with remaining knowledge gaps in this field.
Keywords: cardiogenic shock, COVID‐19, cytokine storm, heart failure, inflammation, multisystemic inflammatory syndrome in adults, myocarditis
Subject Categories: Inflammatory Heart Disease
Nonstandard Abbreviations and Acronyms
- KD
Kawasaki disease
- MIS
multisystemic inflammatory syndrome
- MIS‐A
multisystemic inflammatory syndrome in adults
- MIS‐C
multisystemic inflammatory syndrome in children
- NLRP3
nucleotide‐binding domain, leucine‐rich–containing family, pyrin domain‐containing‐3
Multisystemic inflammatory syndrome (MIS) is a rare but severe postinfectious syndrome that has been described in children (MIS in children [MIS‐C]) as a Kawasaki‐like syndrome following SARS‐CoV‐2 infection. 1 Further identified also in adults (MIS in adults [MIS‐A]), MIS develops as a hyperinflammatory response, leading to multiorgan involvement. 1 Clinical features of MIS‐A overlap considerably with those of MIS‐C, except for the higher incidence of thrombosis, the higher mortality rate, and the severity of cardiac involvement seen in adults. 1 , 2 , 3 , 4 Cardiac involvement in MIS‐A may lead to different clinical scenarios, including arrhythmias, myocarditis, pericarditis, pericardial effusion, and coronary aneurysm. 2 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 However, the pathogenesis of cardiac involvement in MIS‐A is still unknown, and evidence supporting the clinical and pharmacologic management is sparse.
We herein review the clinical features and diagnostic challenges of cardiac manifestations in MIS‐A, discussing possible pathogenetic mechanisms and current evidence on clinical and pharmacologic management.
Search Criteria
This narrative review includes original articles and reviews published on PubMed up to February 2023 using the following terms (or combination of terms): multisystem inflammatory syndrome, MIS‐A, adult, Sars‐CoV‐2, hyperinflammatory syndrome, cytokine storm, and COVID‐19. The inclusion criteria were as follows: (1) case reports, case series, and systematic reviews addressing the prevalence, clinical manifestations, diagnosis, and therapeutic management of patients with MIS and cardiac involvement; (2) patients aged ≥16 years; and (3) patients undergoing a comprehensive diagnostic workup to reach the diagnosis of MIS‐A with cardiac involvement. Exclusion criteria were as follows: (1) studies including patients in whom a temporal association with SARS‐CoV‐2 was not found; and (2) patients aged <16 years (Figure S1).
Clinical Manifestation and Pathophysiology of Cardiac Involvement in MIS‐A
In adults (aged >21 years), MIS‐A presents within 4 to 12 weeks after a SARS‐CoV‐2 infection. 1 The incidence of MIS‐A is still unknown, as most clinical data available are sparse (Table 1). 2 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 In the largest cohort of cases published to date, Patel et al described 221 patients with MIS‐A seen from May 2020 to May 2021. Patients with MIS‐A were relatively young (median age, 21 years), and with no underlying comorbidity in most cases (58%). Most patients were males (70% versus 30% females) with a high prevalence of non‐Hispanic Black individuals (36%). 2 Clinical features of MIS‐A are heterogeneous, including persistent fever associated with gastrointestinal symptoms (pain, vomiting, and diarrhea), signs of mucocutaneous inflammation (annular macular rash or nonpurulent conjunctivitis), hematological disorders (eg, lymphopenia, neutrophilia, and thrombocytopenia), coagulation disorders (arterial and venous thrombosis), and high levels of circulating inflammatory biomarkers (CRP [C‐reactive protein], interleukin [IL]‐6, and ferritin) (Table 2). The cardiovascular system is frequently involved: a greater proportion of patients with MIS‐A may present with cardiac dysfunction and myocarditis compared with those with MIS‐C (114/210 [54%] versus 975/3362 [29%], and 61/205 [30%] versus 543/3639 [15%], respectively). 2 Despite the young age, patients with MIS‐A experience severe complications, including cardiogenic and/or distributive shock (110 of 214 [51%]), admission to the intensive care unit (115 of 201 [57%]), and respiratory support (101 of 213 [47%], of whom 53 [52%] needed mechanical ventilation), with a larger proportion of deaths compared with what was observed in patients with MIS‐C (15 of 220 [7%] versus 27 of 3639 [1%]). 2 , 16 However, it is unknown whether MIS‐A is underreported compared with MIS‐C, and patients with milder disease who did not seek medical advice may account for these discrepancies between MIS‐A and MIS‐C complication rates.
Table 1.
Case Reports and Case Series of MIS‐A With Cardiac Involvement
| Study | Sample size | Sex, n (%) | Age, y | Cardiac dysfunction (EF ≤50%), n (%) | Myocarditis, n (%) | Death, n (%) | Immunomodulatory treatment, n (%) |
|---|---|---|---|---|---|---|---|
| Patel et al 2 | 221 |
Male: 154 (70) Female: 65 (30) |
21 (Median) IQR 19–35 |
114 (54) | 61 (30) | 1 (7) |
IVIG: 112 (55) Corticosteroids: 152 (74) Immune modulators (anakinra and tocilizumab): 42 (21) |
| Gurin et al 5 | 1 | Male | 26 | 1 (100) | 1 (100) | 0 (0) |
IVIG Corticosteroids |
| Shen et al 6 | 1 | Male | 43 | 1 (100) | 1 (100) | 0 (0) | IVIG |
| Patel et al 7 | 1 | Male | 30 | 1 (100) | 1 (100) | 0 (0) | Corticosteroids |
| Morris et al 8 | 16 |
Male: 7 (44) Female: 9 (56) |
21–50 | 9 (56) | 0 (0) | 2 (13) |
Corticosteroids: 10 (62) IVIG: 7 (44) Tocilizumab: 2 (13) |
| Hékimian et al 9 | 11 |
Male: 6 (55) Female: 5 (45) |
16–40 | 11 (100) | 6 (55) | 1 (9) |
IVIG: 5 (45) Corticosteroids: 4 (76) |
| Vannella et al 10 | 1 | Male | 26 | 1 (100) | 1 (100) | 1 (100) | None |
| Chau et al 11 | 7 | Male: 7 (100) | 20–42 | 7 (100) | 0 (0) | 0 (0) | Corticosteroids: 7 (100) |
| Cattaneo et al 12 | 1 | Male | 27 | 1 (100) | 0 (0) | 0 (0) |
Corticosteroids Anakinra |
| Veyseh et al 13 | 1 | Male | 43 | 1 (100) | 0 (0) | 0 (0) | Corticosteroids |
| Diakite et al 14 | 1 | Male | 33 | 1 (100) | 1 (100) | 0 (0) |
IVIG Corticosteroids |
| Aldeghaither et al 15 | 3 | Male: 2 (67) Female: 1 (33) | 21–39 | 3 (100) | 3 (100) | 0 (0) |
Corticosteroids: 3 (100) IVIG: 2 (67) Anakinra: 2 (67) |
EF indicates ejection fraction; IQR, interquartile range; IVIG, intravenous immunoglobulin; and MIS‐A, multisystemic inflammatory syndrome in adults.
Table 2.
Centers for Disease Control and Prevention Diagnostic Criteria of MIS‐A
3. Meet laboratory evidence criteria.
|
CRP indicates C‐reactive protein; LVEF, left ventricular ejection fraction; MIS‐A, multisystemic inflammatory syndrome in adults; and RT‐PCR, reverse transcription–polymerase chain reaction.
The pathophysiology of MIS‐A and its cardiac manifestations is unknown. Postinfectious immune dysregulation is implicated, given that most patients dramatically improve with immunomodulatory agents (Figure 1). 2 , 3 , 4 , 5 , 6 , 8 , 12 A macrophage hyperactivation, leading to a hyperinflammatory state and to a cytokine storm, has been described in this syndrome, with the activation of the IL‐1/IL‐6 downstream pathway playing a pivotal role in the activation of the innate immune response. 17 , 18 , 19 There are 2 biologically active forms of IL‐1: IL‐1α and IL‐1β. IL‐1α is an alarmin that is released into the extracellular space in cell death by necrosis, 20 whereas, IL‐1β is actively produced by inflammatory cells and needs to be activated from the pro‐IL‐1β form. 21 The processing of the pro‐IL‐1β is provided by the caspase‐1 enzymes, which can be activated through a macromolecular structure predominantly expressed in the macrophages known as the nucleotide‐binding domain, leucine‐rich–containing family, pyrin domain–containing‐3 (NLRP3) inflammasome. The NLRP3 inflammasome is implicated in many cardiovascular diseases, including acute myocardial infarction and heart failure, and inflammatory diseases, such as myopericarditis and cardiac sarcoidosis. 22 , 23 , 24 , 25 , 26 , 27 In MIS‐C, the NLRP3 inflammasome pathway was shown to be activated by damage‐associated molecular patterns, as its circulating transcription products are elevated during the acute phase of the disease. 28 Similarly, it is reasonable that the NLRP3 inflammasome and IL‐1β signal could play a role also in the pathophysiology of MIS‐A with cardiac involvement, representing a potential therapeutic target for this condition. The release of damage‐associated molecular patterns and pathogen‐associated molecular pattern molecules leads also to the activation of the cellular component of the innate immune response, including macrophages and dendritic cells, that in turn enhances the secretion of other proinflammatory cytokines, such as the IL‐6. IL‐6 promotes the synthesis of acute‐phase proteins (eg, CRP and serum ferritin) by the liver, which initiates the activation of the adaptative immune response by stimulating the effector T‐cell development and antibody production, with the aim of clearing the viral infection from spreading systemically. However, high levels of IL‐6 secretion were shown also to contribute to the immune response dysregulation in different infectious and hyperinflammatory conditions, including Kawasaki disease (KD) and SARS‐CoV‐2 infection, where increased levels of IL‐6 have been associated with adverse clinical outcomes (intensive care unit admission, severe pulmonary involvement, and death). 29 , 30 In addition, elevated levels of the acute‐phase proteins, CRP and serum ferritin, were shown to correlate with disease severity (shock, intensive care unit admission, and death) in children and adults, thus highlighting the relevance of acute inflammation in the pathophysiology of this syndrome. 31 , 32 , 33 , 34
Figure 1. Main pathophysiological mechanisms implicated in multisystemic inflammatory syndrome in adults (MIS‐A) with cardiac involvement.
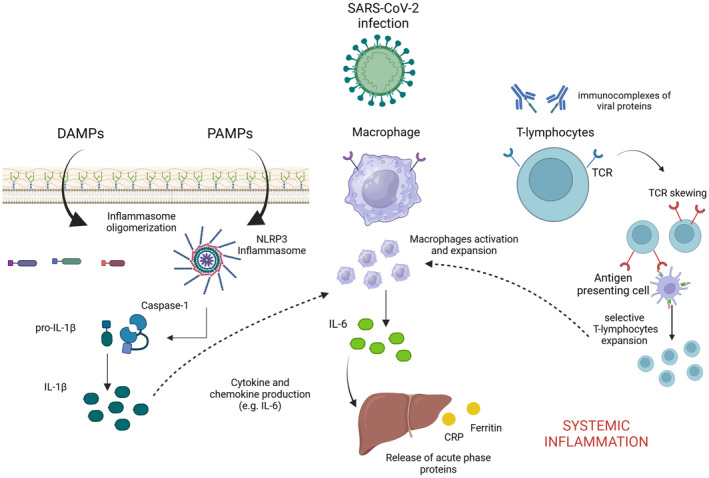
Immune dysregulation is a key pathogenetic mechanism underlying MIS‐A with cardiac involvement. Both adaptative and innate responses are involved in this process. Immune innate response is activated by viral damage‐associated molecular patterns (DAMPs) and pathogen‐associated molecular patterns (PAMPs) that induce nucleotide‐binding domain, leucine‐rich–containing family, pyrin domain‐containing (NLRP) inflammasome activation and interleukin (IL)‐1β production from its proactive form by caspase‐1. IL‐1β is responsible for the production of chemokines and cytokines, such as IL‐6. IL‐6 is produced mainly by activated macrophages and dendritic cells and may induce the production of different acute‐phase response proteins (eg, CRP [C‐reactive protein] and ferritin), initiating the systemic spread of the inflammatory process. Finally, the adaptative immune response is also involved. Indeed, circulating viral proteins included in immune complexes may elicit T‐cell receptor (TCR) skewing, which leads to a rearrangement of the TCR molecular process that gives the T lymphocyte a specific selectivity to recognize the major histocompatibility complex–bound proteins, causing nonspecific T‐cell activation and massive cytokine release responsible for the hyperinflammatory state.
Case reports of T‐cell–mediated myocarditis in patients affected by MIS‐A after SARS‐CoV‐2 infection suggested a potential contribution of the adaptive immune response in the pathogenesis of cardiac injury. 10 Cross‐reactivity of viral epitopes with tissue‐specific self‐antigens, virus‐encoded superantigens driving T‐cell activation, and the formation of immune complexes may be involved in the development of cardiac involvement even in the absence of active in situ viral replication. Indeed, postmortem histopathologic analyses did not demonstrate RNA of the SARS‐CoV‐2 in the cardiac tissue of these patients. 10 Circulating immunocomplexes of spike proteins have been isolated in patients affected by MIS‐C, suggesting the possibility of a superantigen‐like response. According to this hypothesis, a viral domain of the SARS‐CoV‐2 protein engaged in circulating immunocomplexes may elicit the immune system to produce T‐cell receptor skewing, a molecular process that leads to a rearrangement of the surface T‐cell receptor, giving the T lymphocyte a specific selectivity to recognize the major histocompatibility complex–bound proteins that cause a nonspecific polyclonal T‐cell activation and massive cytokine release responsible for the hyperinflammatory state. 35 , 36 , 37 The SARS‐CoV‐2 spike proteins have been isolated in a free (unbound to antibodies) circulating form, in young adults developing post–COVID‐19 mRNA vaccine myocarditis. 38 Hence, the presence of the circulating viral protein has been considered a biomarker of immune dysregulation following the mRNA vaccine, rather than a causative agent of cardiac involvement. 38 However, whether this hypothesis can be verified in adults is unclear, considering the differences in how adults respond to mRNA vaccination compared with adolescents. 38 , 39
Other hyperinflammatory diseases share similar clinical features of MIS, including KD, KD shock syndrome, adult‐onset Still disease, and the macrophage activation syndrome. KD is a postinfectious pediatric vasculitis involving medium‐sized vessels, which can lead to different cardiovascular manifestations, including coronary artery dilatation and aneurysm. 29 In the acute phase, ≈7% of patients with KD may present with hemodynamic instability, a condition known as KD shock syndrome. Despite some differences in demographic and laboratory findings (patients with MIS are generally older, and had higher white blood cell counts and CRP, fibrinogen, and troponin levels), the similarity in clinical features and the development of coronary artery aneurysms in both disorders may represent a key point for the future understanding of pathophysiologic mechanisms of MIS. Adult‐onset Still disease is a systemic inflammatory disorder affecting primarily young adults, which can present with high spiking fever, high levels of inflammatory biomarkers (ie, CRP, erythrocyte sedimentation rate, IL‐6, and ferritin), and, rarely, cardiac involvement (pericarditis, myocarditis, and left ventricular [LV]/right ventricular systolic dysfunction have also been described). 40 Clinical features help to differentiate adult‐onset Still disease from MIS‐A, including the evidence of arthritis, pharyngitis, lymphadenopathy, an evanescent salmon‐colored skin rash, appearing on the trunk and the extremities during febrile episodes, along with the laboratory evidence of leukocytosis, with at least 80% granulocytes. Adult‐onset Still disease can be rarely complicated by macrophage activation syndrome, a severe hyperinflammatory disease presenting with fever, lymphadenopathy, hepatosplenomegaly, encephalopathy, and disseminated intravascular coagulation, also described in systemic juvenile idiopathic arthritis, systemic lupus erythematosus, and MIS. 40 , 41 High levels of circulating biomarkers of inflammation (ie, serum ferritin) are observed in patients affected by macrophage activation syndrome, along with laboratory evidence of pancytopenia, hypertriglyceridemia, hypofibrinogenemia, coagulopathy, and elevated liver function test results. Bone marrow biopsy is usually required to confirm the diagnosis by revealing phagocytosis of blood cell precursors by activated macrophages. 41 , 42 , 43
MIS‐A and Cardiovascular Complications
Clinical features of MIS may significantly overlap with those of different hyperinflammatory and cardiovascular diseases; therefore, the diagnostic workup is challenging (Table 3). The Brighton Collaboration MIS‐C Working Group proposed a case definition of MIS‐C/MIS‐A (Table 2). 1 Clinical features include evidence of fever (subjective fever or documented fever [≥38.0° C]) and ≥2 clinical findings of systemic inflammation (mucocutaneous, gastrointestinal, and neurologic symptoms, and hypotension/shock). Also, patients with MIS‐A should have the following: (1) evidence of current or previous (within 12 weeks) SARS‐CoV‐2 infection; and (2) signs of significant systemic inflammation, coagulopathy, or both. 1
Table 3.
Differential Diagnosis of MIS‐A
| Variable | MIS‐A | Kawasaki disease | MAS | AOSD |
|---|---|---|---|---|
| Clinical features of systemic inflammation | High fever with or without systemic vasodilation/distributive shock | Fever with or without systemic vasodilation/distributive shock (KDSS) | Intermittent/nonremittent fever | High (>39.0° C) spiking fever |
| Cardiac manifestations | Myocarditis, pericarditis, coronary artery dilatation/aneurysm, new‐onset LV/RV dysfunction (LVEF <50%), tachy‐brady arrhythmias | Coronary aneurysms (usually more frequent than in MIS‐A) | New‐onset LV/RV systolic dysfunction (LVEF <50%), pericarditis | New‐onset LV/RV systolic dysfunction (LVEF <50%), pericarditis, myocarditis |
| Other clinical findings | Gastrointestinal symptoms (abdominal pain, vomiting, or diarrhea), rash, nonpurulent conjunctivitis, neurologic symptoms (encephalopathy, seizures, meningeal signs, or peripheral neuropathy) | Oral mucositis, conjunctivitis, rash, cervical adenopathy, hand and foot swelling, late fingertip desquamation | Hepatosplenomegaly, lymphadenopathy, arthritis, encephalopathy, and disseminated intravascular coagulation | Arthritis, pharyngitis, evanescent, salmon‐colored rash, lymphadenopathy |
| Laboratory tests | IL‐6 >1.8 pg/mL, ferritin >300 ng/mL (men) or >150 ng/mL (women), fibrinogen >400 mg/dL, NT‐proBNP >125 ng/L, troponin I elevated, CRP >10 mg/dL, procalcitonin <0.05 ng/mL, erythrocyte sedimentation rate >20 mm/h, lymphocytes <1000×109/L, platelet count <150×109/L, and positive COVID‐19 test via RT‐PCR, serology analysis, or antigen detection |
Leukocytosis (>11×109/L) with neutrophilia (>80% or >7500×109/L), platelet count >500×109/L, ↑CRP >10 mg/dL, procalcitonin <0.05 ng/mL, erythrocyte sedimentation rate >20 mm/h, fibrinogen >400 mg/dL, NT‐proBNP >125 ng/L, troponin I elevated, slightly elevated serum transaminase |
Ferritin level >684 ng/mL plus any 2 of the following: pancytopenia (platelet count ≤181×109/L, hemoglobin <90 g/L, neutrophils <1.0×109/L), aspartate aminotransferase >48 units/L, triglyceride concentration >156 mg/dL, or fibrinogen ≤360 mg/dL, and hemophagocytosis in bone marrow, spleen, or lymph nodes |
Leukocytosis (>11×109/L) with neutrophilia (>80% or >7500×109/L), ferritin >300 ng/mL (men) or >150 ng/mL (women), CRP >10 mg/dL, procalcitonin <0.05 ng/mL, erythrocyte sedimentation rate >20 mm/h |
| Cardiac imaging findings |
Echocardiography: LV/RV dysfunction, MR, pericardial effusion CMR: LV/RV systolic dysfunction with edema, nonischemic fibrosis, pericarditis |
Echocardiography: LV/RV wall motion abnormalities following the coronary artery distribution. CMR: LV/RV systolic dysfunction with edema, ischemic fibrosis, inducible myocardial ischemia (stress CMR) |
Echocardiography: LV/RV dysfunction, pericardial effusion CMR: LV/RV systolic dysfunction with edema, nonischemic fibrosis, pericarditis |
Echocardiography: LV/RV systolic dysfunction, pericardial effusion |
AOSD indicates adult‐onset Still disease; CMR, cardiac MR; CRP, C‐reactive protein; IL‐6, interleukin 6; KDSS, Kawasaki disease shock syndrome; LV, left ventricular; LVEF, LV ejection fraction; MAS, macrophage activation syndrome; MIS‐A, multisystemic inflammatory syndrome in adults; MR, magnetic resonance; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; RT‐PCR, reverse transcription–polymerase chain reaction; and RV, right ventricular.
Cardiac involvement has been considered under the clinical definition of MIS, and includes elevation of cardiac biomarkers, electrocardiographic changes, or imaging findings of cardiac disease 1 (Figure 2). Serum biomarkers of cardiac injury (troponin I, brain natriuretic peptide, and NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide]) are often elevated in patients affected by MIS‐A. 2
Figure 2. Differential diagnosis, diagnostic workup, risk assessment, and therapeutic management of multisystemic inflammatory syndrome in adults (MIS‐A) with cardiac involvement.
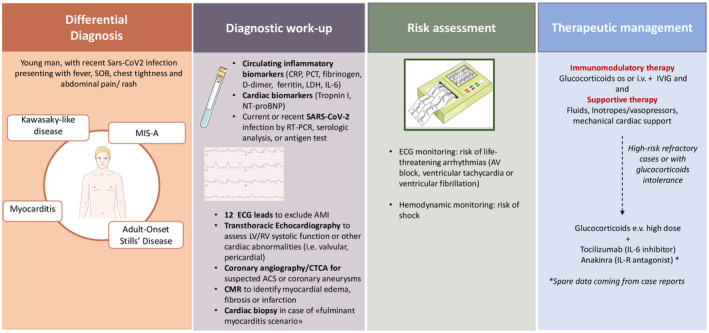
ACS indicates acute coronary syndrome; AMI, acute myocardial infarction; AV, atrioventricular; CMR, cardiac magnetic resonance; CRP, C‐reactive protein; CTCA, computed tomography coronary angiography; IL, interleukin; i.v., intravenous; IVIG, i.v. immunoglobulin; LDH, lactate dehydrogenase; LV, left ventricular; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; os, orally; PCT, procalcitonin; RV, right ventricular; SOB, shortness of breath; and RT‐PCR, reverse transcription–polymerase chain reaction.
The electrocardiographic changes are nonspecific and may be consistent with myocarditis (abnormal ST segments or low‐voltage QRS in the presence of edema), pericarditis (PR segment depression), ischemia (abnormal ST segments, T‐wave inversion), or necrosis (pathologic Q waves). 1 , 2 Moreover, particularly in the presence of a myocarditis‐like scenario, life‐threatening ventricular arrhythmias, such as ventricular tachycardia or ventricular fibrillation, have been documented. 2 Other possible arrhythmic manifestations of MIS‐A include supraventricular tachyarrhythmias (atrial fibrillation or atrial flutter) and atrioventricular or intraventricular conduction delay. 2 When MIS‐A with cardiac involvement is suspected, the assessment of myocardial function should always be performed. Transthoracic echocardiography is the most common and simple diagnostic tool to use and may show right or left ventricular systolic dysfunction (also assessed through ventricular myocardial speckle strain imaging), regional wall motion abnormalities, valvular abnormalities (mitral regurgitation), or pericardial effusion. Perivascular echocardiographic brightness may also suggest the possibility of coronary involvement as in KD‐like phenotypes, requiring further assessment through computed tomography coronary angiography or coronary angiogram. 1
Cardiac magnetic resonance (CMR) may provide more precise quantification of myocardial function, additional information on myocardial tissue characterization, and concomitant complications (ie, pericardial effusion, mitral regurgitation). In particular, CMR may confirm the diagnosis of myocarditis in the presence of T2‐based markers for myocardial edema and T1‐based markers for associated myocardial injury (late gadolinium enhancement, increased native myocardial T1 relation time, or extracellular volume), according to the updated Lake Louise criteria recommended for the CMR diagnosis of myocarditis. 44 In patients with MIS‐A with myocardial dysfunction or a myocarditis‐like scenario, CMR studies showed diffuse myocardial edema as well as focal subepicardial or intramural cardiac edema, with or without evidence of fibrosis at late gadolinium enhancement sequences. 2 , 6 , 7 , 8 , 9 , 10
Coronary angiography may be required to rule out the diagnosis of acute myocardial infarction attributable to atherosclerotic coronary artery disease, according to the cardiovascular risk profile of each patient and the clinical presentation. Moreover, it may highlight coronary abnormalities (eg, dilation, aneurysm, and lack of distal tapering) that have been included in the case definitions of MIS‐A. 1
In addition, in patients presenting with myocarditis considered at high risk, defined on LV dysfunction severity and with hemodynamic or electric instability, endomyocardial biopsy may be recommended to confirm the diagnosis, define the histopathologic pattern, and guide clinicians in setting up the optimal therapeutic strategy. 10 Histopathologic studies in patients with MIS‐A are sparse, documenting different types of cell infiltrates in the absence of the SARS‐CoV‐2 RNA in the cardiac tissue of these patients. 5 , 8 , 10 , 15 , 45 In most cases, an extensive inflammatory infiltrate with predominant interstitial macrophages and T lymphocytes has been described. 5 , 10 , 15 , 45 Few case series also reported a patchy interstitial myocardial eosinophilic and lymphocytic infiltrate, consistent with the diagnosis of eosinophilic myocarditis. Some cases of myocarditis‐like presentation were not confirmed histologically. 45
Pharmacologic Management and Prognosis
Evidence on the pharmacologic treatment of MIS‐A with cardiac involvement is mostly based on case reports and case series. Given the pathogenesis of this hyperinflammatory syndrome, experts achieved consensus about the need to initiate an immunomodulation therapy, including glucocorticoids, intravenous immunoglobulin (IVIG), or cytokine‐targeted therapy, as soon as possible, to limit the course of the illness in addition to hemodynamic pharmacologic or mechanical supportive therapy 1 , 5 , 9 (Table 4). However, in the absence of randomized clinical trials, a consensus around specific immunomodulatory therapies or their possible combination is lacking.
Table 4.
Medical Treatment of MIS‐A With Cardiac Involvement
| Category | Glucocorticoids | Immunoglobulin | Anakinra | Tocilizumab |
|---|---|---|---|---|
| Population | All patients without contraindications | As corticosteroid‐sparing therapy or in combination as first‐line therapy | Patients with moderate to severe disease resistant to glucocorticoids | Patients with moderate to severe disease resistant to glucocorticoids |
| Medication and dose use |
|
2 g/kg in 1 or 4 divided infusions within the first ten days from the disease onset alone or in combination with glucocorticoids | 100 mg daily by subcutaneous injection. Consider higher (often intravenous) doses, up to 10 mg/kg daily in cases of severe hyperinflammation |
8 mg/kg intravenously (to a maximum of 800 mg). If clinical improvement does not occur within 24 h after, up to 3 additional doses may be administered |
| Mechanism of action | Glucocorticoids produce anti‐inflammatory and immunosuppressive effects through inhibition of proinflammatory cytokines and chemokines and inhibition of T and B cells | Immunoglobulin accelerates the clearance of circulating immunocomplexes, promotes the inhibition of complement deposition, and promotes the enhancement of regulatory T cells | Anakinra is a recombinant human IL‐1 receptor antagonist that promotes the downregulation of the downstream cascade events secondary to IL‐1 release | Tocilizumab inhibits the IL‐6 receptor and prevents the downstream activation of the IL‐6 pathway, the differentiation of CD4+ T cells, and the inhibition of T‐regulatory cells |
| Adverse effects | Hyperglycemia and iatrogenic diabetes, psychiatric disturbances, immunosuppression, hypertension, peptic ulcer, osteoporosis, weight gain, adrenal suppression | Fever, dermatological adverse effects, arrhythmia, neutropenia, prothrombotic effects | Increased risk of serious infection, hepatitis, injection‐site reactions | Increased risk of serious infection, gastrointestinal perforations, neutropenia, and thrombocytopenia, increased level of hepatic indexes |
| Contraindications | Systemic fungal infection, diabetes with poor glycemic control, known hypersensitivity, osteoporosis, glaucoma, joint infections | Active infective disease, known hypersensitivity, IgA‐deficient patients | Active infective disease, known hypersensitivity | Active infective disease, known hypersensitivity |
CS indicates cardiogenic shock; IgA, immunoglobulin A; IL‐1, interleukin 1; IL‐6, interleukin 6; and MIS‐A, multisystemic inflammatory syndrome in adults.
Intravenous glucocorticoids should be initiated as soon as other potential conflicting differential diagnoses have been ruled out. Indeed, using glucocorticoids has resulted in rapid recovery in patients presenting with severe features of MIS‐C. 46 Moreover, they are commonly used in KD, where they were shown to improve coronary artery abnormalities and decrease the duration of symptoms in patients with high‐risk features. 29 Low‐dose glucocorticoids may be used in patients with low‐ to moderate‐risk disease (methylprednisolone intravenously or orally 0.3–0.4 mg/d to be gradually tapered), whereas high doses (methylprednisolone intravenously up to 1 g intravenously for 3 days, followed by 1–2 mg/kg to be tapered over 8–12 weeks) should be considered in refractory cases, including cardiogenic shock or fulminant myocarditis, as recommended by international consensus documents for the therapeutic management of acute myocarditis. 5 , 6 , 8 , 9 , 10 , 11 , 12 , 47
IVIG proved to be successful in different case series of MIS‐A and should be considered as a first‐line therapy. 5 , 6 , 7 , 9 The mechanism of action of IVIG includes accelerated clearance of circulating immunocomplexes, inhibition of complement deposition, and enhancement of regulatory T cells. Consensus documents and current guidelines support the use of IVIG administered at a dose of 2 g/kg in 1 or 4 divided infusions every 6 hours in patients with cardiac dysfunction and in patients with MIS‐C within the first ten days from the disease onset (class I, level of evidence A). 46 IVIG can be used alone or with low‐to‐moderate glucocorticoids in patients with clinical features of high‐risk disease. 46 , 48 , 49 , 50 , 51 However, clinical evidence derived from randomized clinical trials supporting their use in MIS‐A is lacking, and no levels of evidence or class of recommendation is still available. Other immunomodulatory agents are reasonable in patients who cannot tolerate glucocorticoids and IVIG, or in patients who clinically worsen despite treatment with these therapies. Sparse data coming from case reports documented the efficacy of biological agents in this setting.
Anakinra (Kineret) is a recombinant human IL‐1 receptor antagonist that downregulates the downstream cascade events secondary to IL‐1 release (both IL‐1α and IL‐1β). 43 Anakinra has been used with glucocorticoids or in glucocorticoid‐dependent severe cases of MIS‐C/MIS‐A, showing favorable outcomes. 1 , 22 , 43 , 52 , 53 The adult dose is 100 mg daily by subcutaneous injection. However, anakinra is often used at higher (often intravenous) doses, up to 10 mg/kg daily in cases of severe hyperinflammation. 54 Anakinra represents a glucocorticoid‐sparing strategy for MIS, because of its efficacy, good safety profile, and short half‐life. Anakinra has also been shown to reduce the progression of pneumonia and mortality in patients with COVID‐19 pneumonia. 55
In case of no clinical improvement after an adequate dose of anakinra within 24 to 48 hours of treatment, other targeted immunomodulatory agents can be considered. Case reports documented the efficacy of tocilizumab, a recombinant IL‐6 receptor antagonist, which has been studied for the treatment of the hyperinflammatory state following the SARS‐CoV‐2 infection. 56 , 57 , 58 The inhibition of IL‐6 prevents the downstream activation of the IL‐6 pathway, the differentiation of CD4+ T cells, and the inhibition of T‐regulatory cells, thus preserving the homeostasis of immunologic tolerance. 59 The efficacy of tocilizumab has been already documented in the treatment of MIS‐C after a single intravenous dose administration. 42 , 60 , 61 On the other hand, data supporting its use in adults affected by MIS‐A are sparse and based on few case reports. 1 , 2 , 8
Cardiac manifestations of MIS‐C often improve or normalize before hospital discharge, and ≈20% of those with coronary artery aneurysm and 7% to 9% of those with LV dysfunction showed residual cardiac lesions at short‐term follow‐up. 1 , 17 , 62 However, persistent abnormalities in strain and diastolic function in patients with MIS‐C and normal LV ejection fraction have been documented, suggesting that a subclinical myocardial injury may persist. 62 To date, short‐ and long‐term follow‐up data in adults are lacking. However, according to clinical presentation, it is essential to warrant an adequate clinical and imaging follow‐up for these patients after the hospital discharge.
Laboratory testing should be monitored to document the normalization of inflammatory markers and resolution of hematologic abnormalities and to guide glucocorticoid tapering. Echocardiogram should be obtained at regular intervals for evaluation of LV ejection fraction, and should ideally include strain analysis, to underpin subclinical structural persistent abnormalities. Moreover, CMR may be considered at 3 to 6 months for a further assessment of systolic function, edema, and scar burden in those patients presenting with myocarditis. ECGs (and possibly 24‐hour electrocardiographic monitoring) should also be repeated to monitor the arrhythmic burden. 2
Prophylactic anticoagulation (in the absence of an indication for full‐dose anticoagulation [ie, atrial fibrillation, mechanical valve, and cardiac thrombi]) should always be considered because of the hypercoagulable state associated with the hyperinflammatory state, especially for patients who are hospitalized and with high‐risk features. 63 There is no consensus on the length of this treatment, which should be established according to the thrombotic versus hemorrhagic risk profile and to the resolution of the hyperinflammatory state based on laboratory findings (eg, CRP, ferritin).
Conclusions
MIS is a recently recognized rare condition attributable to a dysregulated hyperinflammatory state following an infectious disease in children and adults, with a large incidence of cardiac involvement and possible severe, life‐threatening complications. Despite the description of MIS‐C, data about MIS‐A are limited. This syndrome has been described following SARS‐CoV‐2 infection, but it is reasonable to speculate that it may also occur following other viral infections. The pathophysiology and treatment of MIS‐A with cardiac involvement are still based on case series and, in the absence of randomized clinical trials, there is a lack of evidence on how and when to use specific immune‐modulatory therapies. Moreover, in children, the cardiac involvement has proved to be mostly reversible, with a full recovery of the ventricular function before discharge; midterm and long‐term follow‐up data in adults are still lacking.
Sources of Funding
None.
Disclosures
None.
Supporting information
Figure S1
Drs G. La Vecchia and M.G. Del Buono contributed equally.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032143
For Sources of Funding and Disclosures, see page 9.
References
- 1. Vogel TP, Top KA, Karatzios C, Hilmers DC, Tapia LI, Moceri P, Giovannini‐Chami L, Wood N, Chandler RE, Klein NP, et al. Multisystem inflammatory syndrome in children and adults (MIS‐C/a): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037–3049. doi: 10.1016/j.vaccine.2021.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel P, DeCuir J, Abrams J, Campbell AP, Godfred‐Cato S, Belay ED. Clinical characteristics of multisystem inflammatory syndrome in adults: a systematic review. JAMA Netw Open. 2021;4:e2126456. doi: 10.1001/jamanetworkopen.2021.26456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Minocha PK, Phoon CKL, Verma S, Singh RK. Cardiac findings in pediatric patients with multisystem inflammatory syndrome in children associated with COVID‐19. Clin Pediatr (Phila). 2021;60:119–126. doi: 10.1177/0009922820961771 [DOI] [PubMed] [Google Scholar]
- 4. Alsaied T, Tremoulet AH, Burns JC, Saidi A, Dionne A, Lang SM, Newburger JW, de Ferranti S, Friedman KG. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation. 2021;143:78–88. doi: 10.1161/CIRCULATIONAHA.120.049836 [DOI] [PubMed] [Google Scholar]
- 5. Gurin MI, Lin YJ, Bernard S, Goldberg RI, Narula N, Faillace RT, Alviar CL, Bangalore S, Keller NM. Cardiogenic shock complicating multisystem inflammatory syndrome following COVID‐19 infection: a case report. BMC Cardiovasc Disord. 2021;21:522. doi: 10.1186/s12872-021-02304-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shen M, Milner A, Foppiano Palacios C, Ahmad T. Multisystem inflammatory syndrome in adults (MIS‐A) associated with SARS‐CoV‐2 infection with delayed‐onset myocarditis: case report. Eur Heart J Case Rep. 2021;5:ytab470. doi: 10.1093/ehjcr/ytab470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel K, Mehta R, Betz YM, Man LM. Cardiac complications from multisystem inflammatory syndrome associated with prior COVID‐19 infection. BMJ Case Rep. 2022;15:e249889. doi: 10.1136/bcr-2022-249889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morris SB, Schwartz NG, Patel P, Abbo L, Beauchamps L, Balan S, Lee EH, Paneth‐Pollak R, Geevarughese A, Lash MK, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS‐CoV‐2 infection ‐ United Kingdom and United States, March‐August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450–1456. doi: 10.15585/mmwr.mm6940e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hékimian G, Kerneis M, Zeitouni M, Cohen‐Aubart F, Chommeloux J, Bréchot N, Mathian A, Lebreton G, Schmidt M, Hié M, et al. Coronavirus disease 2019 acute myocarditis and multisystem inflammatory syndrome in adult intensive and cardiac care units. Chest. 2021;159:657–662. doi: 10.1016/j.chest.2020.08.2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vannella KM, Oguz C, Stein SR, Pittaluga S, Dikoglu E, Kanwal A, Ramelli SC, Briese T, Su L, Wu X, et al. Evidence of SARS‐CoV‐2‐specific T‐cell‐mediated myocarditis in a MIS‐A case. Front Immunol. 2021;12:779026. doi: 10.3389/fimmu.2021.779026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chau VQ, Giustino G, Mahmood K, Oliveros E, Neibart E, Oloomi M, Moss N, Mitter SS, Contreras JP, Croft L, et al. Cardiogenic shock and hyperinflammatory syndrome in young males with COVID‐19. Circ Heart Fail. 2020;13:e007485. doi: 10.1161/CIRCHEARTFAILURE.120.007485 [DOI] [PubMed] [Google Scholar]
- 12. Cattaneo P, Volpe A, Cardellino CS, Riccardi N, Bertoli G, Ursini T, Ustalli A, Lodi G, Daroui I, Angheben A. Multisystem inflammatory syndrome in an adult (MIS‐A) successfully treated with anakinra and glucocorticoids. Microorganisms. 2021;9:1393. doi: 10.3390/microorganisms9071393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Veyseh M, Webster P, Blanco I. COVID‐19‐associated inflammatory syndrome in an adult woman with unexplained multiple organ failure: staying vigilant for COVID‐19 complications as the pandemic surges. BMJ Case Rep. 2021;14:e242034. doi: 10.1136/bcr-2021-242034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diakite S, Bousdira N, Tachon G, Ackermann F, Groh M, Rohmer J. Regression of coronary aneurysms with intravenous immunoglobulins and steroids for COVID‐19 adult multisystem inflammatory syndrome. JACC Case Rep. 2021;3:581–585. doi: 10.1016/j.jaccas.2021.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aldeghaither S, Qutob R, Assanangkornchai N, Issa‐Chergui B, Tam M, Larotondo R, Samoukovic G. Clinical and histopathologic features of myocarditis in multisystem inflammatory syndrome (adult)‐associated COVID‐19. Crit Care Explor. 2022;10:e0630. doi: 10.1097/CCE.0000000000000630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, Soma VL, Maddux AB, Mourani PM, Bowens C, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS‐C) compared with severe acute COVID‐19. JAMA. 2021;325:1074–1087. doi: 10.1001/jama.2021.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Webb BJ, Peltan ID, Jensen P, Hoda D, Hunter B, Silver A, Starr N, Buckel W, Grisel N, Hummel E, et al. Clinical criteria for COVID‐19‐associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2:e754–e763. doi: 10.1016/S2665-9913(20)30343-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weisberg SP, Connors TJ, Zhu Y, Baldwin MR, Lin WH, Wontakal S, Szabo PA, Wells SB, Dogra P, Gray J, et al. Distinct antibody responses to SARS‐CoV‐2 in children and adults across the COVID‐19 clinical spectrum. Nat Immunol. 2021;22:25–31. doi: 10.1038/s41590-020-00826-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weatherhead JE, Clark E, Vogel TP, Atmar RL, Kulkarni PA. Inflammatory syndromes associated with SARS‐CoV‐2 infection: dysregulation of the immune response across the age spectrum. J Clin Invest. 2020;130:6194–6197. doi: 10.1172/JCI145301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dinarello CA. Overview of the IL‐1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281:8–27. doi: 10.1111/imr.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim B, Lee Y, Kim E, Kwak A, Ryoo S, Bae SH, Azam T, Kim S, Dinarello CA. The interleukin‐1α precursor is biologically active and is likely a key alarmin in the IL‐1 family of cytokines. Front Immunol. 2013;4:391. doi: 10.3389/fimmu.2013.00391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toldo S, Kannan H, Bussani R, Anzini M, Sonnino C, Sinagra G, Merlo M, Mezzaroma E, De‐Giorgio F, Silvestri F, et al. Formation of the inflammasome in acute myocarditis. Int J Cardiol. 2014;171:e119–e121. doi: 10.1016/j.ijcard.2013.12.137 [DOI] [PubMed] [Google Scholar]
- 23. Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin‐1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res. 2020;126:1260–1280. doi: 10.1161/CIRCRESAHA.120.315937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mezzaroma E, Abbate A, Toldo S. The inflammasome in heart failure. Curr Opin Physio. 2021;19:105–112. doi: 10.1016/j.cophys.2020.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15:203–214. doi: 10.1038/nrcardio.2017.161 [DOI] [PubMed] [Google Scholar]
- 26. Kron J, Mauro AG, Bonaventura A, Toldo S, Salloum FN, Ellenbogen KA, Abbate A. Inflammasome formation in granulomas in cardiac sarcoidosis. Circ Arrhythm Electrophysiol. 2019;12:e007582. doi: 10.1161/CIRCEP.119.007582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vecchié A, Del Buono MG, Chiabrando GJ, Dentali F, Abbate A, Bonaventura A. Interleukin‐1 and the NLRP3 inflammasome in pericardial disease. Curr Cardiol Rep. 2021;23:157. doi: 10.1007/s11886-021-01589-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang WT, He M, Shimizu C, Croker BA, Hoffman HM, Tremoulet AH, Burns JC, Shyy JY. Inflammasome activation in children with Kawasaki disease and multisystem inflammatory syndrome. Arterioscler Thromb Vasc Biol. 2021;41:2509–2511. doi: 10.1161/ATVBAHA.121.316210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, et al. Diagnosis, treatment, and long‐term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 30. Coomes EA, Haghbayan H. Interleukin‐6 in Covid‐19: a systematic review and meta‐analysis. Rev Med Virol. 2020;30:1–9. doi: 10.1002/rmv.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao Y, Yin L, Patel J, Tang L, Huang Y. The inflammatory markers of multisystem inflammatory syndrome in children (MIS‐C) and adolescents associated with COVID‐19: a meta‐analysis. J Med Virol. 2021;93:4358–4369. doi: 10.1002/jmv.26951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hortová‐Kohoutková M, Skotáková M, Onyango IG, Slezáková M, Panovský R, Opatřil L, Slanina P, De Zuani M, Mrkva O, Andrejčinová I, et al. Hepcidin and ferritin levels as markers of immune cell activation during septic shock, severe COVID‐19 and sterile inflammation. Front Immunol. 2023;14:1110540. doi: 10.3389/fimmu.2023.1110540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abrams JY, Oster ME, Godfred‐Cato SE, Bryant B, Datta SD, Campbell AP, Leung JW, Tsang CA, Pierce TJ, Kennedy JL, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS‐C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5:323–331. doi: 10.1016/S2352-4642(21)00050-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bartsch YC, Wang C, Zohar T, Fischinger S, Atyeo C, Burke JS, Kang J, Edlow AG, Fasano A, Baden LR, et al. Humoral signatures of protective and pathological SARS‐CoV‐2 infection in children. Nat Med. 2021;27:454–462. doi: 10.1038/s41591-021-01263-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boribong BP, LaSalle TJ, Bartsch YC, Ellett F, Loiselle ME, Davis JP, Gonye ALK, Sykes DB, Hajizadeh S, Kreuzer J, et al. Neutrophil profiles of pediatric COVID‐19 and multisystem inflammatory syndrome in children. Cell Rep Med. 2022;3:100848. doi: 10.1016/j.xcrm.2022.100848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Porritt RA, Paschold L, Rivas MN, Cheng MH, Yonker LM, Chandnani H, Lopez M, Simnica D, Schultheiß C, Santiskulvong C, et al. HLA class I‐associated expansion of TRBV11‐2 T cells in multisystem inflammatory syndrome in children. J Clin Invest. 2021;131:e146614. doi: 10.1172/JCI146614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yonker LM, Swank Z, Bartsch YC, Burns MD, Kane A, Boribong BP, Davis JP, Loiselle M, Novak T, Senussi Y, et al. Circulating spike protein detected in post‐COVID‐19 mRNA vaccine myocarditis. Circulation. 2023;147:867–876. doi: 10.1161/CIRCULATIONAHA.122.061025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ogata AF, Cheng CA, Desjardins M, Senussi Y, Sherman AC, Powell M, Novack L, Von S, Li X, Baden LR, et al. Circulating severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccine antigen detected in the plasma of mRNA‐1273 vaccine recipients. Clin Infect Dis. 2022;74:715–718. doi: 10.1093/cid/ciab465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still's disease. J Autoimmun. 2018;93:24–36. doi: 10.1016/j.jaut.2018.07.018 [DOI] [PubMed] [Google Scholar]
- 41. Hines MR, von Bahr Greenwood T, Beutel G, Beutel K, Hays JA, Horne A, Janka G, Jordan MB, van Laar JAM, Lachmann G, et al. Consensus‐based guidelines for the recognition, diagnosis, and management of hemophagocytic lymphohistiocytosis in critically ill children and adults. Crit Care Med. 2022;50:860–872. doi: 10.1097/CCM.0000000000005361 [DOI] [PubMed] [Google Scholar]
- 42. Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, Behrens EM, Ferris A, Kernan KF, Schulert GS, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS‐CoV‐2 and hyperinflammation in pediatric COVID‐19: version 2. Arthritis Rheumatol. 2021;73:e13–e29. doi: 10.1002/art.41616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rodriguez‐Smith JJ, Verweyen EL, Clay GM, Esteban YM, de Loizaga SR, Baker EJ, Do T, Dhakal S, Lang SM, Grom AA, et al. Inflammatory biomarkers in COVID‐19‐associated multisystem inflammatory syndrome in children, Kawasaki disease, and macrophage activation syndrome: a cohort study. Lancet Rheumatol. 2021;3:e574–e584. doi: 10.1016/S2665-9913(21)00139-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ferreira VM, Schulz‐Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 45. Bemtgen X, Klingel K, Hufnagel M, Janda A, Bode C, Staudacher DL, Supady A, Jandova I. Case report: lymphohistiocytic myocarditis with severe cardiogenic shock requiring mechanical cardiocirculatory support in multisystem inflammatory syndrome following SARS‐CoV‐2 infection. Front Cardiovasc Med. 2021;8:716198. doi: 10.3389/fcvm.2021.716198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, Grimaud M, Oualha M, Beghetti M, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS‐CoV‐2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360 [DOI] [PubMed] [Google Scholar]
- 47. Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, Friedrich MG, Klingel K, Lehtonen J, Moslehi JJ, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020;13:e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang D, Fu B, Peng Z, Yang D, Han M, Li M, Yang Y, Yang T, Sun L, Li W, et al. Tocilizumab in patients with moderate or severe COVID‐19: a randomized, controlled, open‐label, multicenter trial. Front Med. 2021;15:486–494. doi: 10.1007/s11684-020-0824-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kaushik S, Aydin SI, Derespina KR, Bansal PB, Kowalsky S, Trachtman R, Gillen JK, Perez MM, Soshnick SH, Conway EE Jr, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS‐C): a multi‐institutional study from New York City. J Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sood M, Sharma S, Sood I, Sharma K, Kaushik A. Emerging evidence on multisystem inflammatory syndrome in children associated with SARS‐CoV‐2 infection: a systematic review with meta‐analysis. SN Compr Clin Med. 2021;3:38–47. doi: 10.1007/s42399-020-00690-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kest H, Kaushik A, DeBruin W, Colletti M, Goldberg D. Multisystem inflammatory syndrome in children (MIS‐C) associated with 2019 novel coronavirus (SARS‐CoV‐2) infection. Case Rep Pediatr. 2020;2020:8875987. doi: 10.1155/2020/8875987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rinaldi E, Tomelleri A, Campochiaro C, Dagna L. Efficacy of interleukin‐1 blockade with anakinra in the management of post‐COVID‐19 steroid‐dependent multisystem inflammatory syndrome: a case report. Scand J Rheumatol. 2023;52:230–232. doi: 10.1080/03009742.2022.2140485 [DOI] [PubMed] [Google Scholar]
- 53. Cavalli G, De Luca G, Campochiaro C, Della‐Torre E, Ripa M, Canetti D, Oltolini C, Castiglioni B, Tassan Din C, Boffini N, et al. Interleukin‐1 blockade with high‐dose anakinra in patients with COVID‐19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fisher CJ Jr, Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ, Greenman RL, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double‐blind, placebo‐controlled trial. Phase III rhIL‐1ra Sepsis Syndrome Study Group. JAMA. 1994;271:1836–1843. [PubMed] [Google Scholar]
- 55. Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, Fragkou A, Rapti A, Damoulari C, Fantoni M, et al. Early treatment of COVID‐19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double‐blind, randomized controlled phase 3 trial. Nat Med. 2021;27:1752–1760. doi: 10.1038/s41591-021-01499-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Soin AS, Kumar K, Choudhary NS, Sharma P, Mehta Y, Kataria S, Govil D, Deswal V, Chaudhry D, Singh PK, et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID‐19‐associated cytokine release syndrome (COVINTOC): an open‐label, multicentre, randomised, controlled, phase 3 trial. Lancet Respir Med. 2021;9:511–521. doi: 10.1016/S2213-2600(21)00081-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, Bruzzi P, Boni F, Braglia L, Turrà C, et al. Effect of Tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID‐19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg‐Yoo A, Schenck EJ, Radbel J, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID‐19. JAMA Intern Med. 2021;181:41–51. doi: 10.1001/jamainternmed.2020.6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sheppard M, Laskou F, Stapleton PP, Hadavi S, Dasgupta B. Tocilizuma (Actemra). Hum Vaccin Immunother. 2017;13:1972–1988. doi: 10.1080/21645515.2017.1316909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schlapbach LJ, Andre MC, Grazioli S, Schöbi N, Ritz N, Aebi C, Agyeman P, Albisetti M, Bailey DGN, Berger C, et al; PIMS‐TS working group of the Interest Group for Pediatric Neonatal Intensive Care (IGPNI) of the Swiss Society of Intensive Care and the Pediatric Infectious Diseases Group Switzerland (PIGS). Best practice recommendations for the diagnosis and management of children with Pediatric Inflammatory Multisystem Syndrome temporally associated with SARS‐CoV‐2 (PIMS‐TS; multisystem inflammatory syndrome in children, MIS‐C) in Switzerland. Front Pediatr. 2021;9:667507. doi: 10.3389/fped.2021.667507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hoste L, Van Paemel R, Haerynck F. Multisystem inflammatory syndrome in children related to COVID‐19: a systematic review. Eur J Pediatr. 2021;180:2019–2034. doi: 10.1007/s00431-021-03993-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matsubara D, Kauffman HL, Wang Y, Calderon‐Anyosa R, Nadaraj S, Elias MD, White TJ, Torowicz DL, Yubbu P, Giglia TM, et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID‐19 in the United States. J Am Coll Cardiol. 2020;76:1947–1961. doi: 10.1016/j.jacc.2020.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID‐19. J Thromb Haemost. 2020;18:1559–1561. doi: 10.1111/jth.14849 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


