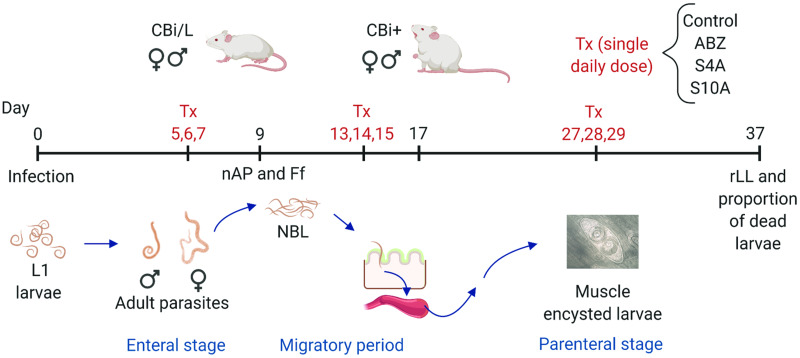
Fig. 1.
Graph outlining the experimental design used. CBi+ and CBi/L mice of both sexes infected with T. spiralis infective L1 larvae were divided into three groups to receive the treatment (Tx) in the enteral (days 5, 6, 7 p-i), migratory (days 13, 14, 15 p-i), or parenteral (days 27, 28, 29 p-i) stages of T. spiralis infection. Within each group, mice were subdivided into four subgroups according to the formulation given daily as a single oral dose (30 mg ABZ/kg body weight/day): control, ABZ, S4A and S10A. Efficacy of the treatments was evaluated by determining the intestinal worm load (nAP) and T. spiralis female fecundity (Ff), and muscle relative Larval Load (rLL) and proportion of dead larvae in the tongue.