Fig. 1.
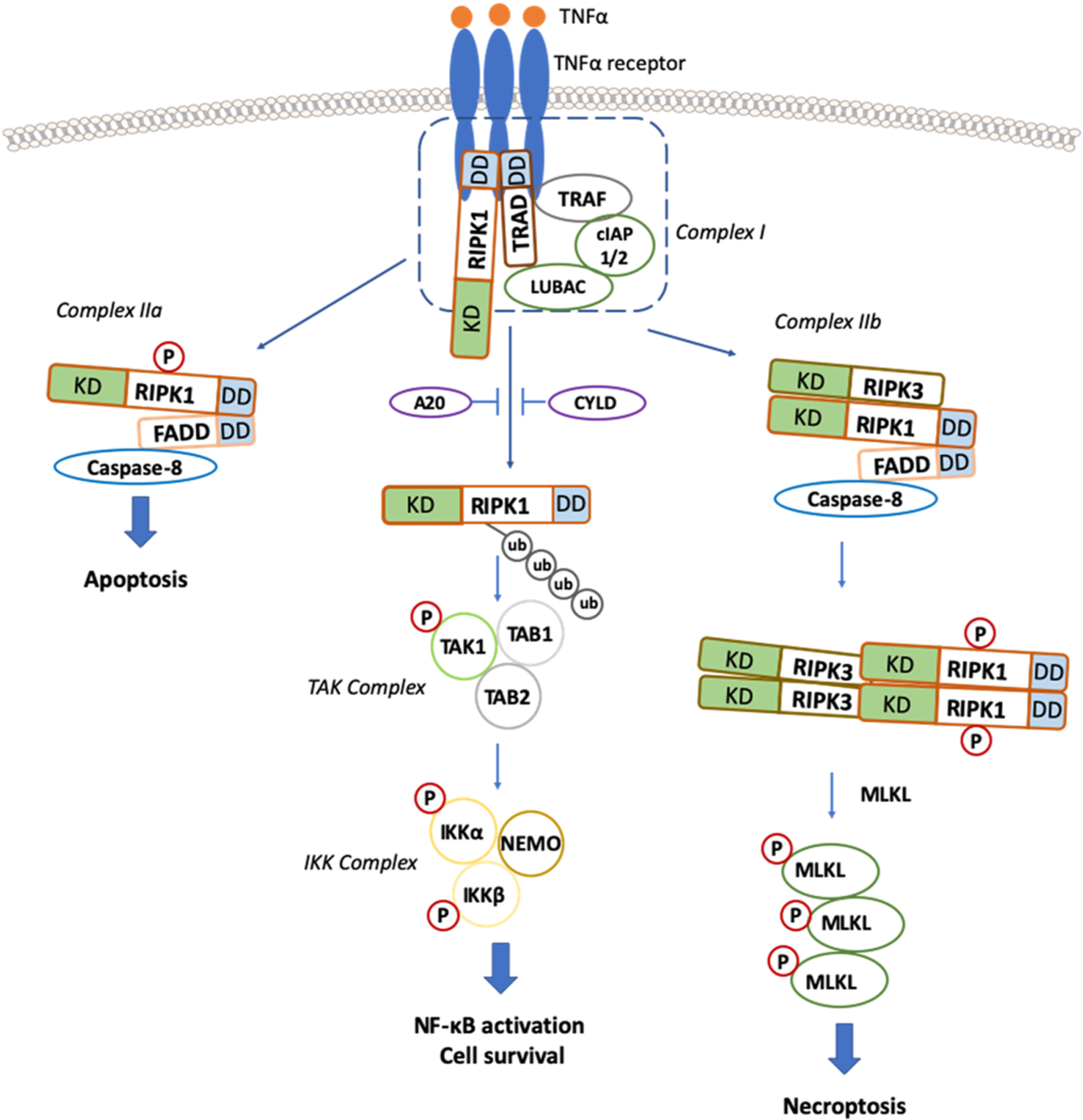
Canonical TNF/TNFR1 dependent Necroptosis Signaling Cascades. Upon TNF-α stimulation, the activated TNF receptor (TNFR) interacts with TNFR1-associated death domain protein (TRADD), TRAFs, and RIPK1 and recruits cIAP1 and cIAP2 to form a plasma membrane-associated complex, resulting in RIPK1 polyubiquitination. Inhibition of cIAPs (by SMAC or SMAC mimetics) leads to deubiquitination of RIPK1 and dissociation of RIPK1 from the complex. RIPK1 then binds to FADD and caspase-8 to form a complex called complex IIa, which activates caspase-8 and leads to apoptosis induction. When caspase-8 activity is blocked, RIPK1 binds to RIPK3 to form a complex IIb or necrosome in a RIPK1-kinase-dependent manner. Then, RIPK3 undergoes auto-phosphorylated and subsequent activation, allowing RIPK3 to recruit and phosphorylate MLKL. The translocation of phosphorylated MLKL to the plasma membrane promotes necroptosis by disrupting plasma and intracellular membrane integrity. KD: kinase domain, DD: death domain.
