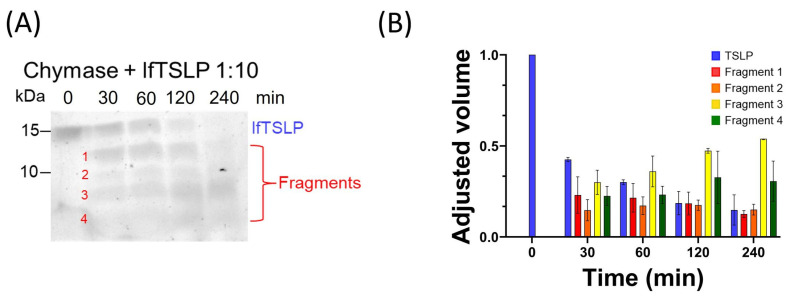
Figure 2.
Cleavage analysis of lfTSLP by chymase. (A) Recombinant human TSLP (5 μg) was treated with chymase (0.5 μg at 37 °C). 1 μg aliquots were withdrawn at 0, 30, 60, 120, and 240 min, inactivated by heating for 10 min at 99 °C to stop the cleavage reaction and separated on 16% Tris-Tricine gel. The gel was stained with colloidal Coomassie Brilliant Blue solution. (B) Densitometric analysis of the cleavage products of TSLP generated by chymase (as shown in panel A). The progressive and marked reduction in the band intensity at ~15 kDa, and the appearance of several smaller fragments, indicated that TSLP is a substrate for chymase. The results show the mean ± SD of 3 independent experiments.