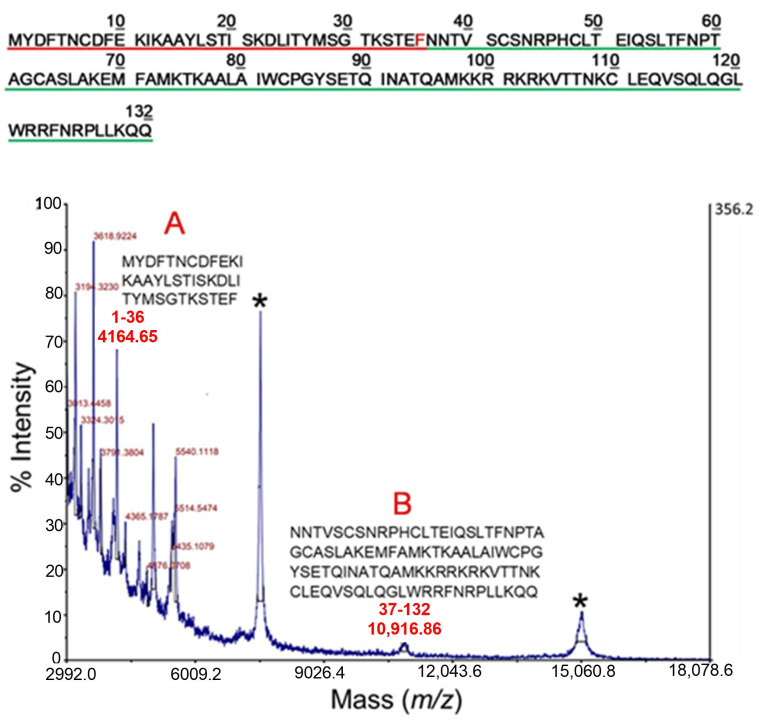
Figure 4.
MALDI-MS analysis of TSLP following incubation with chymase under strictly controlled conditions (E:S 1:100 for 30 min at 37 °C). The signals marked with an asterisk correspond to the mono and doubly charged ions of the intact protein. Peaks at m/z 4164.65 and 10,916.86 were assigned to the complementary peptides 1-36 and 37-132, respectively (marked A and B in the figure) originating from a single proteolytic cleavage at Phe36. The amino acid sequences of the two peptides (A and B) are shown in the inset and are underlined in red (A) or in green (B) in the upper panel of the figure. The chymase preferential cleavage site between Phe36-Asn37 is shown in red. All other peaks in the spectrum were identified as sub-digestion products. Other fragments were observed at lower m/z, indicating further proteolytic cleavage of the two main fragments 1-36 and 37-132.