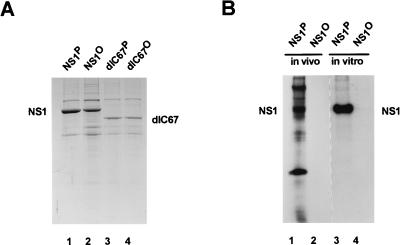
FIG. 2.
Production, dephosphorylation, and purification of NS1. (A) Full-length (wild-type) and C-terminally truncated (dlC67) NS1 were produced by recombinant vaccinia viruses in HeLa cells. Nuclear extracts containing NS1 were treated (dephosphorylated samples, with superscripted “O”) or not treated (native samples, with superscripted “P”) with calf intestine alkaline phosphatase, and the NS1 polypeptides were purified by affinity chromatography by means of their N-terminal [His]6 tags. The purified samples were analyzed by SDS-PAGE and Coomassie blue staining. Lanes: 1 and 2, wild-type NS1; 3 and 4, NS1dlC67; 1 and 3, native NS1; 2 and 4, dephosphorylated NS1. (B) Wild-type NS1 produced by recombinant vaccinia viruses was metabolically 32P labeled in vivo, using 106 HeLa cells, and extracted by repeated freezing and thawing. Alternatively, dephosphorylated NS1 was 32P labeled in vitro, using PKC. The 32P-labeled proteins were then mixed with the same amount of NS1-containing nuclear extract as used in panel A. Phosphatase-treated or untreated samples were then purified on Ni2+-NTA–agarose columns and analyzed by SDS-PAGE and autoradiography. Lanes: 1 and 2, NS1 labeled in vivo in HeLa cells; 3 and 4, NS1 labeled in vitro, using recombinant PKC; 1 and 3, mock treatment; 2 and 4, treatment with calf intestine alkaline phosphatase. The low degree of purification of in vivo 32P-labeled NS1 in lane 1 was probably due to the formation of aggregates caused by the freezing and thawing procedure used to release the 32P-labeled proteins from the cells.