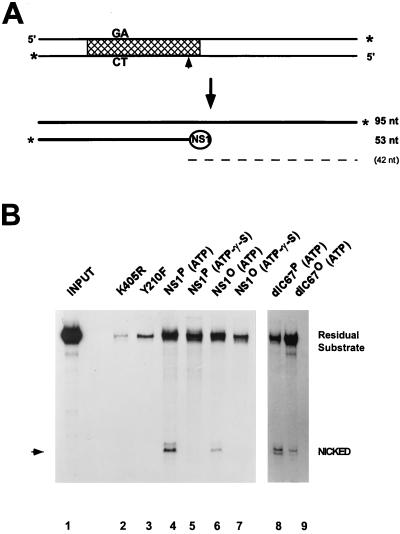
FIG. 4.
Site-specific nicking of native versus dephosphorylated wild-type NS1 and NS1dlC67. (A) Diagram of substrate and denatured products of the nicking reaction. Labeled 3′ ends are marked by asterisks, the cross-hatched area delineates the minimal active left-end origin (16), and the nick site is indicated by an arrowhead. The circled NS1 depicts the covalently linked NS1 at the 5′ end of the nicked strand. The dashed line indicates the predicted unlabeled 42-nucleotide (nt) single-strand product DNA. (B) For each assay, 3′-end-labeled substrate was incubated with purified NS1 in the presence of ATP or γ-S-ATP as indicated. DNA which became covalently attached to NS1 was then immunoprecipitated with αNSN, deproteinized, and analyzed by 8% sequencing gel electrophoresis. The migration of the nicked product is indicated by an arrowhead. NS1 mutants K405R and Y210F served as negative controls. Lanes: 1, input substrate (1:20 dilution); 2 and 3, negative controls, using mutant NS1; 4 to 7, wild-type NS1; 8 and 9, NS1dlC67; 2 to 4, 6, 8, and 9, reactions in the presence of 2 mM ATP; 5 and 7, reactions in the presence of ATP-γ-S. Residual substrate consists of the unnicked positive strand coimmunoprecipitated with the NS1-attached nicked strand, as well as contaminating substrate DNA which was not removed by the washing procedure.