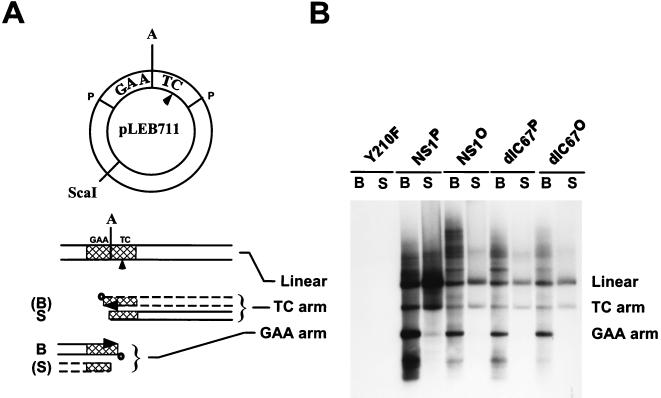
FIG. 7.
In vitro resolution of the 3′ dimer bridge by native and dephosphorylated wild-type NS1 and NS1dlC67 in fully competent HeLa cell replication extracts. (A) Diagram of the plasmid substrate pLEB711 (17, 22) and the resolution products. The MVM dimer bridge, derived from a dimer replication intermediate as a PstI fragment (P), is marked GAA and TC across the axis of symmetry A, according to the sequence corresponding to the unpaired bubble in the left-end palindrome. The dimer bridge is cross-hatched in the reaction products. The nick site, as determined by Cotmore and Tattersall (16), is marked with an arrowhead, and the ScaI restriction site used to linearize the replication products is indicated. The resolution of the dimer bridge is asymmetric and gives rise to major (solid lines) and minor (dashed lines) products (for details, see references 17, 19, and 22). B, NS1 (boldfaced “donut”)-bound extended forms derived from nicking and strand displacement synthesis across the axis of symmetry; S, NS1-free covalently closed turnaround forms after resolution. (B) The 3′ dimer bridge containing plasmid pLEB711 was subjected to resolution and replication in fully competent HeLa cell replication extracts in the presence of dNTPs (including [α-32P]dATP), ATP, and an ATP regeneration system, using native or dephosphorylated wild-type NS1 and NS1dlC67. The reactions were stopped by adding 0.2% SDS and heating at 70°C for 30 min to disrupt noncovalent binding of NS1 to the replicated DNA. The newly synthesized 32P-labeled DNA was then immunoprecipitated with αNSN and digested with ScaI, allowing the resolved NS1-attached (B) products and NS1-free (S) products to be isolated and analyzed separately by 1% agarose gel electrophoresis and autoradiography. The linearized unresolved plasmid, labeled either by nonspecific nick translation (S) or by rolling-circle replication and incomplete resolution (B) of the circular plasmid, as well as the complete resolution products (TC and GAA arms) are indicated. As reported previously, in vitro resolution of pLEB711 by NS1 is asymmetric, producing predominantly the extended form of the GAA arm (NS1 bound) and the turnaround form of the TC arm (NS1 free).