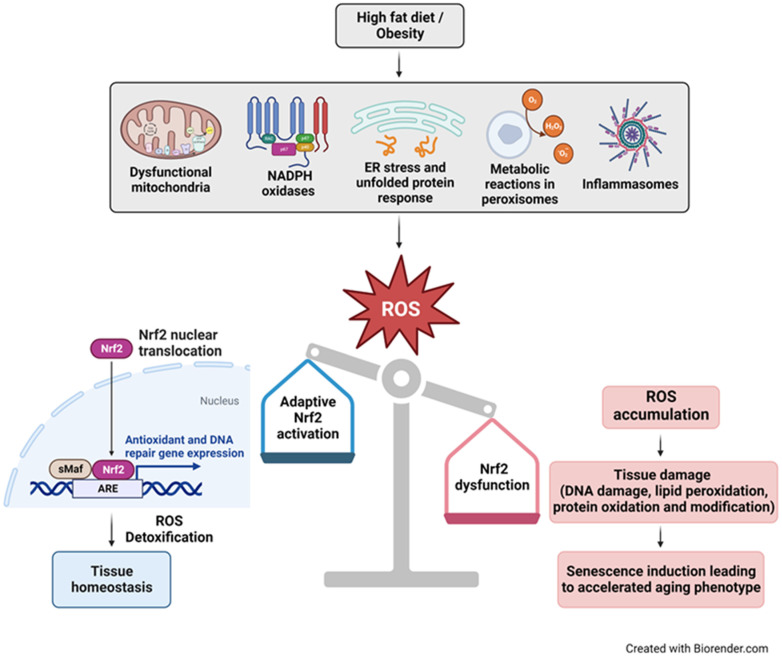
Figure 5.
Schematic illustration of the relationship between consumption of HFD/obesity, oxidative stress, Nrf2 deficiency, and cellular senescence in accelerated aging. Dysfunctional mitochondria, NADPH oxidases, ER stress from misfolded proteins, metabolic processes in peroxisomes, and inflammasomes are major sources of increased reactive oxygen species (ROS) levels in obesity. Nrf2 plays a crucial role in maintaining cellular redox homeostasis. Under conditions of oxidative stress, Nrf2 is released from the Keap1−Cul3−RBX1 complex, after which it translocates into the nucleus, forms heterodimers with small Maf proteins (sMaf), and binds to antioxidant response elements (AREs). This activation leads to the transcription of genes encoding antioxidant enzymes, DNA repair mechanisms and proteins that confer anti-inflammatory effects, facilitating ROS detoxification and restoration of cellular homeostasis. Conversely, impaired Nrf2 activation results in the accumulation of toxic levels of ROS, inducing macromolecular damage like DNA breaks, and triggering senescence, ultimately leading to an accelerated aging phenotype.