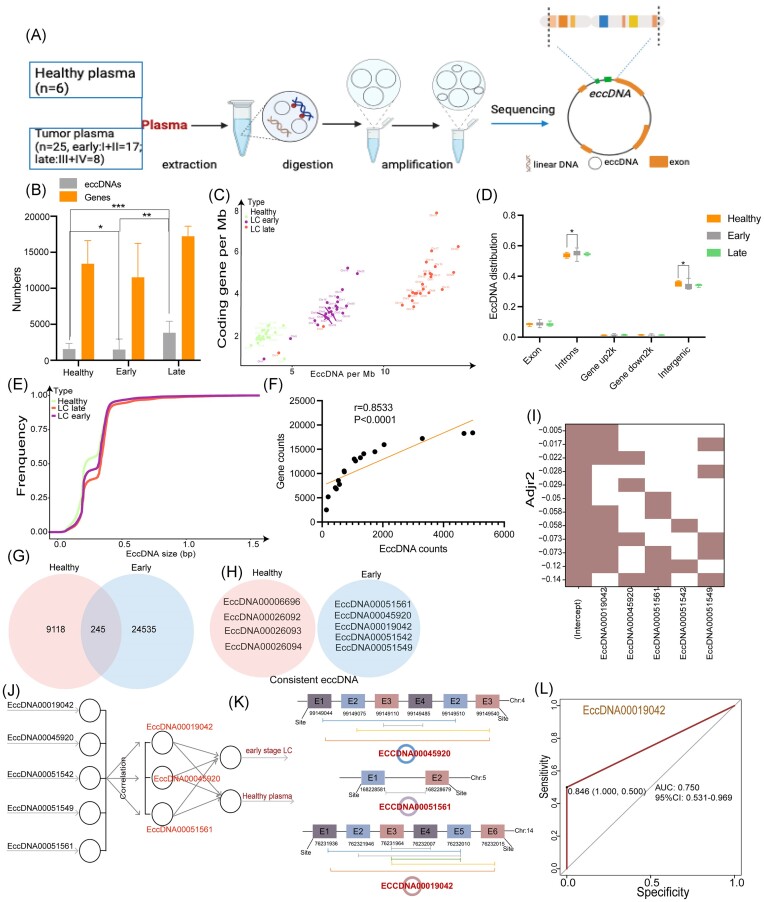
Figure 1.
Identifying potential eccDNAs in early NSCLC plasma samples for diagnosis. (A) The workflow of the processes for detecting eccDNAs in plasma. (B) The counts of eccDNAs and eccDNA genes in samples from healthy controls, early stage and late stage lung cancer patients. (C) The ratios of coding genes/Mb and eccDNAs/Mb of chromosomes in samples from healthy controls, early stage and late stage lung cancer patients. (D) Genomic distributions of eccDNAs in samples from healthy controls, early stage and late stage lung cancer patients. (E) Size distribution of eccDNAs in samples from healthy controls, early stage and late stage lung cancer patients. (F) Pearson correlation analysis between the coding gene/Mb and eccDNA/Mb ratios on chromosomes from healthy controls, early stage and late stage lung cancer patients. (G, H) Consistent eccDNAs and consistent eccDNA genes sequences in tissue samples from healthy controls and early stage lung cancer patients. (I) Heatmap showing the distribution of adjusted R2 values in each model, which was calculated by the leaps R package. (J) The selection workflow for important diagnostic eccDNAs to distinguish early NSCLC from healthy plasma samples. (K) The split sites of ECCDNA00045920 and ECCDNA00051561. (L) ROC curves for EccDNA00019042 in the test dataset (ROC analyses of EccDNA00019042 in early NSCLC and normal plasma samples; early AUC = 0.750, 95% CI = 0.531–0.969).