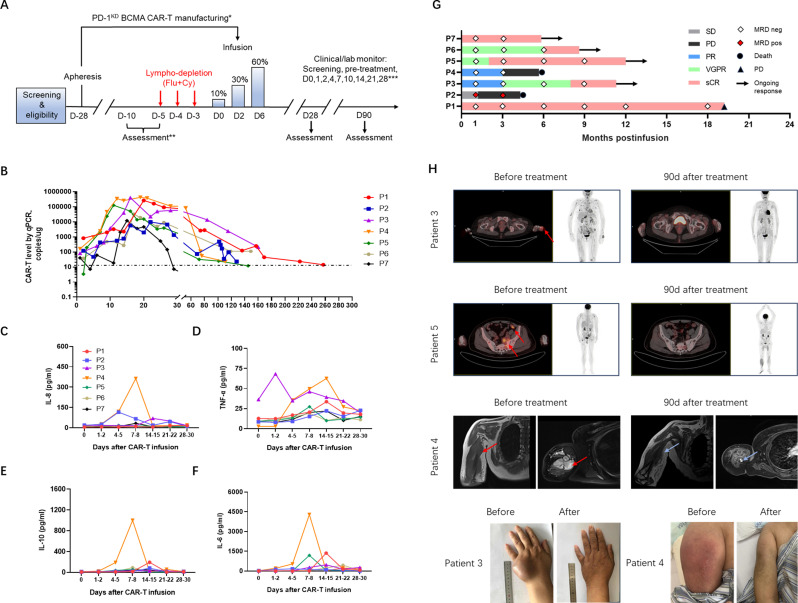
Figure 6.
Clinical efficacy of PD-1KD BCMA CAR-T cells in patients with RRMM. (A) Schematic diagram of the clinical PD-1KD BCMA CAR-T cell treatment regimen and clinical/laboratory monitoring. *Patients may receive therapy during manufacturing to maintain disease control. **After the first 28 days, follow-up was every 4 weeks up to 6 months and then every 3 months up to 2 years. ***Pre-tx, pretreatment, 3–7 days before CAR-T cell infusion. Flu indicates fludarabine. Cy indicates cyclophosphamide. (B) Measurements of CAR-T cells assessed by means of qPCR assay in peripheral blood of patients treated with cyclophosphamide/fludarabine combination conditioning and three-infusion CAR T delivery. (C–F) Cytokine concentrations in the serum of all patients who received infusions of PD-1KD BCMA CAR-T cells, as determined by ELISA. (G) Swimmer plot depicting each subjects’ response category over time and the results of MRD detection via flow cytometry on bone marrow aspirates. (H) Response of extramedullary lesion. Extramedullary infiltration lesions disappeared or were reduced after PD-1KD BCMA CAR-T cell infusion. Representative PET-CT images of patient 3 and patient 5 and MRIs of patient 4 before and after PD-1KD BCMA CAR-T cell treatment. Extramedullary diseases are indicated by red arrows. Light blue arrows indicate tumor reduction. BCMA, B cell maturation antigen; MRD, minimal residual disease; PR, partial response; RRMM, relapsed/refractory multiple myeloma; sCR, stringent complete response; SD, stable disease.