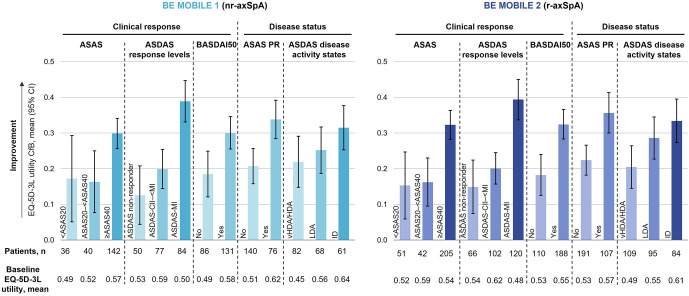
Figure 4.
Associations between clinical composite efficacy endpoints and EQ-5D-3L utility at week 52 (OC). Randomised set. Categories are mutually exclusive. ASDAS reduction less than 1.1 (ie, change from baseline (CfB) >−1.1) is referred to as ASDAS non-responder, ASDAS reduction greater or equal to 1.1 and less than 2.0 (ie, −2.0>CfB ≤−1.1 or ASDAS clinically important improvement (CII), but not major improvement (MI)) is referred to as ASDAS-CII–<MI and ASDAS reduction greater or equal to 2.0 (ie, ASDAS CfB ≤−2.0) is referred to as ASDAS-MI). ASAS, Assessment of SpondyloArthritis International Society; ASAS20, ASAS ≥20% improvement; ASAS40, ASAS ≥40% improvement; ASAS PR, ASAS partial remission; ASDAS, Ankylosing Spondylitis Disease Activity Index; BASDAI50, ≥50% improvement in Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; EQ-5D-3L, European Quality of Life 5 Dimensions 3 Level Version; HDA, high disease activity; ID, inactive disease; LDA, low disease activity; n, number; nr-axSpA, non-radiographic axSpA; OC, observed case; r-axSpA, radiographic axSpA; vHDA, very HDA.