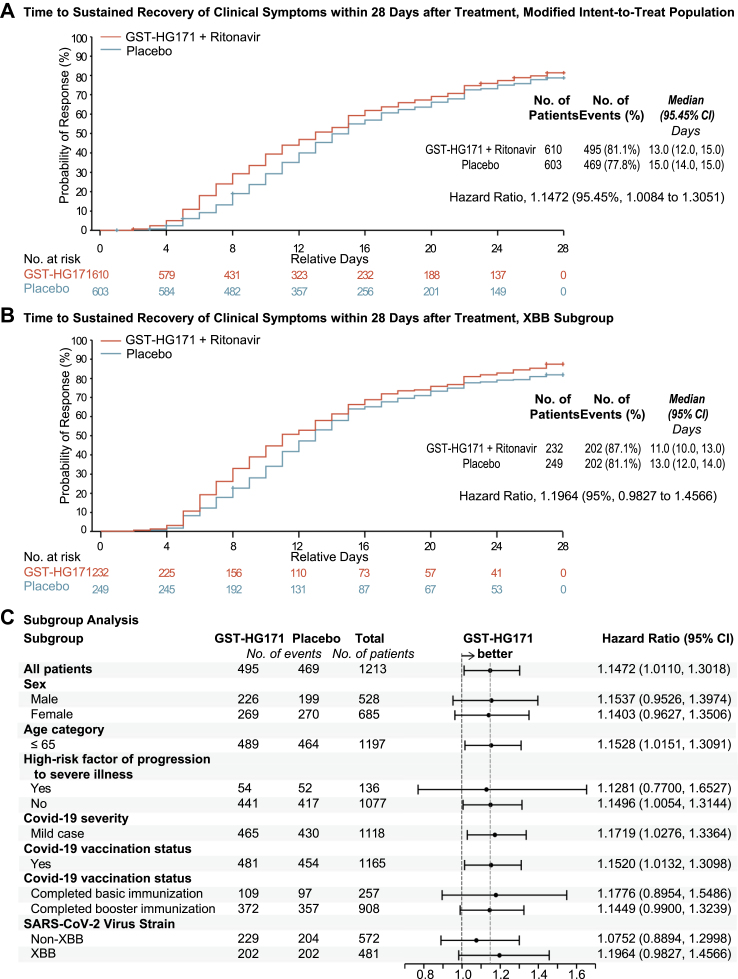
Fig. 2.
Time to Sustained Recovery of Clinical Symptoms. Shown are the time to sustained recovery of clinical symptoms within 28 days after treatment in the mITT population (1213 patients, Panel A) and the subgroup of population that infected with XBB variants (481 patients, Panel B), as estimated with the use of the Kaplan–Meier method and compared with the use of the log-rank test adjusted for randomisation factors, and the hazard ratio (HR, investigational group/placebo group) calculated by the Cox regression model and corrected by randomisation factors. Sustained recovery of clinical symptoms is defined as the score of 0 for all COVID-19-related target symptoms for 2 consecutive days, with the event date defined as the first day of the 2 consecutive days. + indicates censored values. Data for the patients who used prohibited medications or therapies that may affect the efficacy endpoints (as identified at the data review meeting), progressed to severe/critical COVID-19 before recovery, or were assessed by investigators as having poor efficacy and were withdrawn from treatment early, and dead before recovery were censored on Day 28. Panel C shows subgroup analysis of the time to sustained recovery of clinical symptoms within 28 days after treatment in the mITT population, analyzed using the same statistical methods as the primary analysis shown in Panel A. CI, confidence interval; GST-HG171 as a shorthand for GST-HG171 plus Ritonavir.