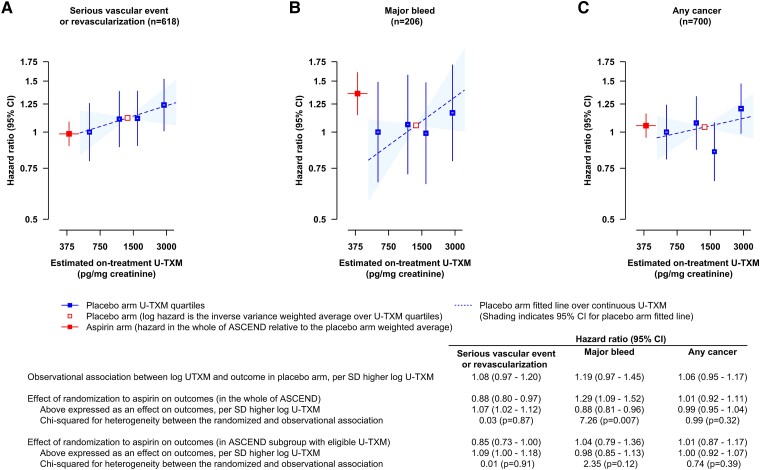
Figure 6.
Comparison of the observational associations of log urinary 11-dehydro-thromboxane B2 with outcomes in the placebo arm and the implied effects in the randomized comparisons of aspirin vs. placebo, if the effect of aspirin on outcomes (A, B and C as in Figure 2) was mediated via its effect on TXA2 biosynthesis as reflected by log urinary 11-dehydro-thromboxane B2. Hazard ratios are plotted at the estimated on-treatment geometric mean urinary 11-dehydro-thromboxane B2, with the placebo groups plotted at the mean baseline log urinary 11-dehydro-thromboxane B2 within quartile (corresponding to geometric means of 601, 1120, 1634, and 2847 pg/mg creatinine). In a randomized comparison of urinary 11-dehydro-thromboxane B2 levels at a mean of 2 years after randomization in aspirin-allocated vs. placebo-allocated participants in ASCEND, aspirin was associated with 1.222 (1.023–1.421, P < .0001) lower log urinary 11-dehydro-thromboxane B2,7 and therefore, the hazard ratio in the aspirin arm relative to the placebo arm was plotted at the mean log urinary 11-dehydro-thromboxane B2 in the placebo arm, 7.193, minus 1.222 = 5.971 (corresponding to 392 pg/mg creatinine). The 95% confidence intervals for the mean urinary 11-dehydro-thromboxane B2 are visible for the aspirin arm but small and within the boxes for the placebo arm. The observational analysis in the placebo arm was from a model including both arms, adjusted for treatment allocation, basic factors and predictors of log urinary 11-dehydro-thromboxane B2 and with different slopes allowed for the hazard ratio per 1 SD of log urinary 11-dehydro-thromboxane B2 in the placebo and aspirin allocated arms. SD of overall baseline log urinary 11-dehydro-thromboxane B2 is 0.622. Hence, 1 SD higher log urinary 11-dehydro-thromboxane B2 corresponds to an exp(0.622) = 1.862 fold higher U-TXM. CI, confidence interval; n, number of events; SD, standard deviation; U-TXM, urinary 11-dehydro-thromboxane B2