Abstract
Uproleselan (GMI-1271) is a novel E-selectin antagonist that disrupts cell survival pathways, enhances chemotherapy response, improves survival in mouse xenograft and syngeneic models, and decreases chemotherapy toxicity in vivo. A phase 1/2 study evaluated the safety, tolerability, and antileukemic activity of uproleselan (5-20 mg/kg) with MEC (mitoxantrone, etoposide, and cytarabine) among patients with relapsed/refractory (R/R) acute myeloid leukemia (AML). Among the first 19 patients, no dose-limiting toxicities were observed. The recommended phase 2 dose (RP2D) was 10 mg/kg twice daily. An additional 47 patients with R/R AML were treated with uproleselan at the RP2D plus MEC. At the RP2D, the remission rate (complete response [CR]/CR with incomplete count recovery [CRi]) was 41% (CR, 35%), and the median overall survival (OS) was 8.8 months. In a separate cohort, 25 newly diagnosed patients age ≥60 years received uproleselan at the RP2D plus cytarabine and idarubicin (7 + 3). In these frontline patients, the CR/CRi rate was 72% (CR, 52%), and the median OS was 12.6 months. The addition of uproleselan was associated with low rates of oral mucositis. E-selectin ligand expression on leukemic blasts was higher in patients with relapsed vs primary refractory AML and in newly diagnosed older patients with high-risk cytogenetics and secondary AML. In the R/R cohort, E-selectin expression >10% was associated with a higher response rate and improved survival. The addition of uproleselan to chemotherapy was well tolerated, with high remission rates, low induction mortality, and low rates of mucositis, providing a strong rationale for phase 3 randomized confirmatory studies. This trial was registered at www.clinicaltrials.gov as #NCT02306291.
Key Points
-
•
Uproleselan at doses ranging from 5 to 20 mg/kg was well tolerated, with adverse event profile similar to that of background chemotherapy.
-
•
Median OS at RP2D (10 mg/kg) in patients with R/R or newly diagnosed AML was 8.8 or 12.6 months, respectively.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous hematologic malignancy of progenitor cell clonal expansion in the peripheral blood and bone marrow. Despite recent improvements, drug resistance and subsequent disease progression dominate the disease course for most patients with AML.1 Patients who are resistant to treatment or who have a short-lived initial response have a poor prognosis, with median overall survival (OS) of 3 to 7 months.2, 3, 4, 5 In addition, despite recent advances,6 treatment of newly diagnosed older patients with AML remains a challenge, with continued poor outcomes resulting from toxicity from induction chemotherapy and short remission durations.
E-selectin expression is induced on vascular endothelial cells and facilitates the extravasation of cells from the bloodstream as part of the inflammatory response.7 E-selectin is constitutively expressed in the bone marrow vasculature, and E-selectin ligands are present on the surface of leukemic blasts and leukemic stem cells (LSCs).8 AML blasts release proinflammatory signals, leading to upregulation of E-selectin on bone marrow endothelium, resulting in enhanced binding of leukemic cells. Binding to E-selectin in the marrow microenvironment sequesters blasts and LSCs in protective niches, inducing a chemotherapy-resistant state.9 This occurs, in part, through activation of the prosurvival, antiapoptotic NF-κB pathway.9, 10
Uproleselan is an E-selectin antagonist that mimics the E-selectin carbohydrate ligand and inhibits the binding of cells to E-selectin, disrupting the leukemic cell adhesion in the bone marrow, thereby abrogating the microenvironment-mediated protection of AML cells.9 In preclinical studies, uproleselan mobilized leukemic cells from the protective niches, blocked NF-κB activation, and enhanced sensitivity to the antileukemic agent cytarabine.9 In mouse xenograft studies, the addition of uproleselan to combination treatment with doxorubicin and cytarabine enhanced survival.9 Accordingly, a phase 1/2 clinical study was conducted in which uproleselan was included in chemotherapy-based induction regimens in patients with relapsed/refractory (R/R) disease and in frontline therapy for older adults with AML.
Methods
Study design
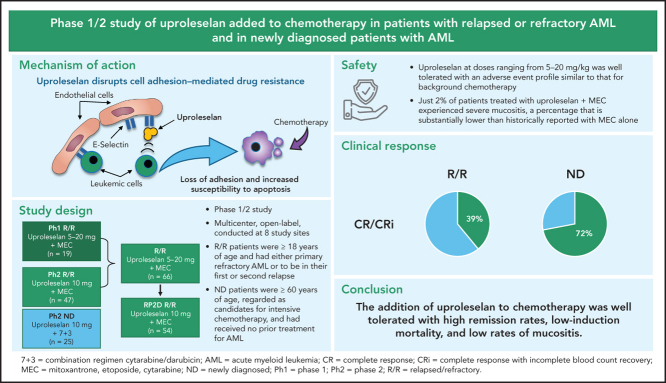
This was a multicenter, open-label, phase 1/2 study conducted at 8 academic hospitals in the United States (6 sites), Ireland (1 site), and Australia (1 site). The initial dose-escalation phase of the study enrolled patients with R/R AML to establish the safety, tolerability, pharmacokinetics (PKs), and recommended phase 2 dose (RP2D) of uproleselan in combination with a conventional salvage chemotherapeutic regimen. The expansion phase of the study evaluated the efficacy of uproleselan at the RP2D in combination with salvage chemotherapy in the same R/R AML population, as well as in combination with a standard (7 + 3) frontline AML induction regimen in a separate cohort of older patients newly diagnosed with AML. The initial dose-escalation phase was monitored by a dose-escalation committee, which was composed of the study investigators and the study medical monitor.
Patients provided written informed consent in accordance with the ethical principles of the Declaration of Helsinki and institutional guidelines at participating sites. The study was conducted under an investigational new drug application in the United States and under clinical trial authorizations in Ireland and Australia and was approved by the relevant institutional review boards or ethics committees. An independent data safety monitoring board provided study oversight, including review of study progress, safety data, and efficacy data at completion of dose escalation and every 6 months.
Patient eligibility
Patients enrolled in the R/R AML cohort in both the dose-escalation and expansion phases were age ≥18 years with evidence of active leukemia as documented by a morphologically detectable bone marrow blast count of ≥5% and a peripheral absolute blast count of ≤20 000/mm3. After evaluation of the first 2 dose cohorts, the allowable peripheral absolute blast count was changed to ≤40 000/mm3. Patients were required to either have primary refractory AML after receiving at least 1, but not >2, prior induction regimens (≥1 containing an anthracycline) or be in their first or second relapse. Patients had to have been eligible to receive an intensive induction regimen including MEC (mitoxantrone, etoposide, and cytarabine; Figure 1). Two cycles of induction therapy using a standard anthracycline/cytarabine regimen (eg, 7 + 3 followed by 5 + 2) were counted as a single induction. Prior stem cell transplantation was allowed. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, with adequate baseline renal and hepatic function. Patients with central nervous system leukemia were ineligible. Antileukemic treatment was prohibited within 14 days of starting study treatment, except for hydroxyurea and FLT-3 or other tyrosine kinase inhibitors, which were allowed up to 5 days before protocol treatment.
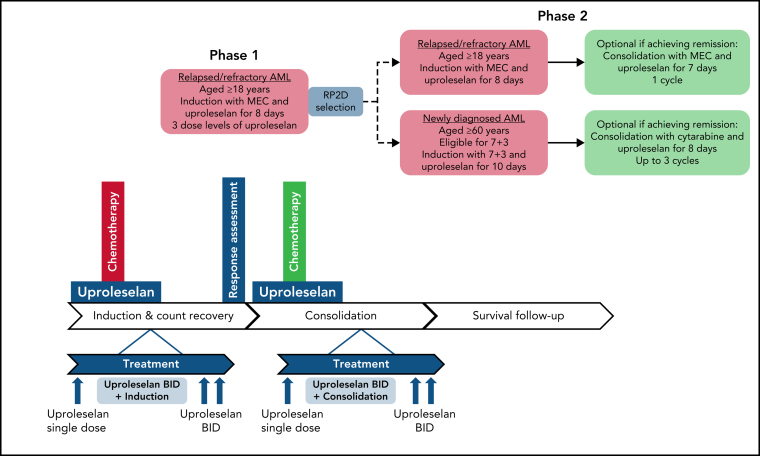
Figure 1.
Treatment schema of phase 1 and phase 2. BID, twice daily; MEC, combination regimen mitoxantrone, etoposide, cytarabine; RP2D, recommended phase 2 dose; 7 + 3, combination regimen cytarabine/idarubicin.
Newly diagnosed patients were enrolled in a separate cohort and were age ≥60 years and regarded as candidates for intensive chemotherapy by their treating physicians. No prior treatment of AML was allowed, except for hydroxyurea. Patients with secondary AML (prior myelodysplastic syndrome, myeloproliferative neoplasm, or therapy-related disease) could receive prior therapy for their antecedent hematologic disorder. Other eligibility criteria for this cohort of patients were the same as those for the R/R cohort of patients.
Treatment
In all study phases, uproleselan was administered as a 20-minute IV infusion given 24 hours prior, twice daily throughout, and twice daily for 48 hours postinduction chemotherapy. The salvage chemotherapy regimen used for patients with R/R disease was MEC (10 mg/m2 of mitoxantrone per day IV over 15-20 minutes, 100 mg/m2 of etoposide per day IV over 60 minutes, and 1000 mg/m2 of cytarabine per day IV over 60 minutes for 5 days) for 1 induction cycle. Uproleselan dose levels were determined by targeting a range of expected exposures, at or above the pharmacologically active dose range, as demonstrated in preclinical models. Based on available PK data from nonhuman primate studies and safety data from human volunteer studies, the starting dose for uproleselan administered with MEC in the dose-escalation phase of the study was 5 mg/kg. Uproleselan was administered across 3 pharmacologically active dose levels (5, 10, and 20 mg/kg). Dose escalation was performed in the absence of dose-limiting toxicity (DLT), defined as myelosuppression (failure of recovery to absolute neutrophil count [ANC] ≥0.5 × 109/L and platelet count of ≥25 × 109/L) beyond day 42 in the absence of persistent morphologic evidence of leukemia in the marrow or grade 3 nonhematologic toxicity attributable to uproleselan and not resolving to grade 2 by day 42. The dose-escalation committee determined an RP2D of 10 mg/kg, defined as the dose that (1) did not cause DLT in >33% of treated patients during induction and (2) was the most appropriate dose for continuing clinical evaluation in AML based on available data (safety, exposure achieved, pharmacodynamic [PD] assessment of on-target effect, clinical activity, and overall toxicity).
The phase 2 dose expansion at the RP2D continued enrolling patients with R/R AML. Responding patients were eligible to receive 1 additional cycle of consolidation with uproleselan combined with a 4-day course of MEC. Alternatively, patients underwent hematopoietic stem cell transplantation and/or received additional postremission therapies at the discretion of their treating physician.
A second cohort of newly diagnosed patients age ≥60 years were enrolled between June 2016 and February 2017 and treated with uproleselan administered at the RP2D in combination with conventional 7 + 3 induction chemotherapy (200 mg/m2 of cytarabine by a 24-hour continuous daily infusion on days 1-7 in combination with 12 mg/m2 of idarubicin by IV bolus daily on days 1-3). Uproleselan was administered on the same schedule as that used in the R/R AML cohort. For patients with residual leukemia detected on a day-15 midcycle bone marrow examination, a second cycle of induction therapy (5 + 2) was allowed in combination with uproleselan at the same dose and schedule as those used during the initial 7 + 3 induction. The first 3 patients were assessed postinduction for DLT; thereafter, enrollment was opened to complete a 25-patient cohort. Responders could receive consolidation therapy with uproleselan plus intermediate-dose cytarabine (2000 mg/m2 per day over 3 hours for 5 days or 1500 mg/m2 over 3 hours every 12 hours on days 1, 3, and 5) for up to 3 cycles. Patients could undergo hematopoietic stem cell transplantation at the discretion of their treating physician.
Safety assessments
The primary end point of the study was the frequency, severity, and relatedness of treatment-emergent adverse events (TEAEs) in patients receiving uproleselan in combination with chemotherapy. Safety assessments included the surveillance and recording of TEAEs, vital sign measurements, clinical laboratory tests, ECOG performance status, and physical examinations. Grade and term of TEAEs were reported by the treating physician and were graded by the Common Terminology Criteria for Adverse Events (version 4.03).
Clinical response and efficacy assessments
Antileukemic activity was assessed by routine laboratory tests, bone marrow examinations, and flow cytometry evaluation of leukemic blasts. Response categorization was based on the 2003 revised recommendations of the International Working Group for AML.11 Bone marrow examinations were performed at baseline (within 21 days of starting treatment) and at the end of induction therapy at the time of count recovery (defined by ANC of ≥1 × 109/L and/or platelet count of ≥100 × 109/L or by day 42 in the absence of count recovery). Patients were monitored for leukemia remission, durability of remission, subsequent treatments, transplantation, and survival.
Statistical analysis
Study measures of safety and activity were summarized by descriptive statistics. The analysis set for all treated patients included those receiving any amount of uproleselan. Time-to-event analyses were performed using the Kaplan-Meier method, with 2-sided 90% confidence intervals (CIs).
Results
Patient characteristics
Between 14 May 2015, and 18 May 2017, 91 patients were enrolled and treated with uproleselan along with chemotherapy in all phases of the study (supplemental Figure 1). Sixty-six patients with R/R AML were enrolled, including 19 patients in the dose-escalation phase and 47 patients in the expansion phase. Including the 7 patients who received 10 mg/kg of uproleselan during the dose-escalation phase, a total of 54 patients were treated with the RP2D of uproleselan in combination with MEC. In the newly diagnosed AML cohort, 25 patients received treatment with 7 + 3 in combination with the RP2D of uproleselan.
R/R AML cohort
Pretreatment characteristics for all patients enrolled in the study are shown in Table 1. The median age of the 66 patients with R/R disease was 59 years (range, 26-84 years). Overall, 33% (22 of 66) of patients entered the study with primary refractory disease, whereas 67% (44 of 66) were in relapse after a previous response. Of patients with relapsed AML, the length of initial remission duration was ≤12 months in 75% (33 of 44) and >12 months in 25% (11 of 44). Overall, 67% (44 of 66) of patients had received 1 prior induction regimen, and 33% (22 of 66) had received ≥2 induction regimens. Twelve patients (18%) had undergone prior hematopoietic stem cell transplantation. A classification of unfavorable-risk cytogenetics (per SWOG criteria) was present in 59% of patients. By ELN criteria, 50% of patients had adverse-risk disease. There was no significant difference in pretreatment variables between the total population of patients with R/R disease and patients treated at the RP2D.
Table 1.
Baseline demographics and characteristics of patients receiving uproleselan
| Characteristic | All doses | 10 mg/kg |
|---|---|---|
| R/R (n = 66) | Newly diagnosed patients age ≥60 y (n = 25) | |
| Sex | ||
| Male | 41 (62) | 14 (56) |
| Female | 25 (38) | 11 (44) |
| Age, y | 59.0 (26-84) | 67.0 (60-79) |
| AML type | ||
| Primary refractory | 22 (33) | — |
| 1 induction regimen | 17/22 (77) | — |
| ≥2 induction regimens | 5/22 (23) | — |
| Relapsed | 44 (67) | — |
| Duration of remission, mo | ||
| ≤6 | 18 (41) | — |
| ≤12 | 33 (75) | — |
| Prior induction regimens, n | ||
| 1 | 44 (67) | — |
| ≥2 | 22 (33) | — |
| Prior hematopoietic stem cell transplantation | 12 (18) | |
| De novo or secondary AML | ||
| De novo | 51 (77) | 12 (48) |
| Secondary | 15 (23) | 13 (52) |
| MDS | 7 (11) | 10 (40) |
| Therapy related | 1 (2) | 1 (4) |
| Other | 7 (11) | 2 (8) |
| ELN risk category | ||
| Favorable | 7 (11) | 3 (12) |
| Intermediate | 11 (17) | 7 (28) |
| Unfavorable | 33 (50) | 12 (48) |
| Unknown | 15 (23) | 3 (12) |
| SWOG risk category | ||
| Favorable | 1 (2) | 1 (4) |
| Intermediate | 24 (36) | 16 (64) |
| Unfavorable | 39 (59) | 8 (32) |
| Unknown | 2 (4) | 0 |
Data are presented as n (%), n/N (%), or median (range).
ELN, European LeukemiaNet; MDS, myelodysplastic syndrome; mo, months; SWOG, Southwest Oncology Group.
Newly diagnosed cohort
The median age of patients with newly diagnosed AML was 67 years (range, 60-79 years). Thirteen (52%) of the 25 newly diagnosed patients had secondary AML at study entry. Seven (28%) of the 25 patients had been treated with hypomethylating agents (HMAs) before progression to AML. A classification of unfavorable cytogenetic risk (per SWOG criteria) was present in 8 (32%) and intermediate risk in 16 (64%) of the 25 newly diagnosed patients. By ELN risk criteria, 48% were in the adverse-risk category, and 28% were intermediate risk.
Safety and tolerability
Dose-escalation cohort
Nineteen patients in the dose-escalation phase were enrolled in 3 separate uproleselan dose-level groups of 5 (n = 6), 10 (n = 7), and 20 mg/kg (n = 6). Uproleselan at 10 mg/kg twice daily was established as the RP2D and was used as the dose for combination therapy with MEC in the dose-expansion phase and with idarubicin and cytarabine in the newly diagnosed cohort. No DLTs were observed at any dose level in phase 1; therefore, DLT did not influence dose-level selection for phase 2. PD analysis demonstrated on-target activity as measured by reduction in shed soluble E-selectin in the plasma at all 3 dose levels. Furthermore, no dose response was observed, suggesting that all 3 dose levels assessed may have exceeded a plateau level for PD effect. Clinical outcomes (bone marrow response to uproleselan with MEC-induction chemotherapy) were similar across the dose levels. Based on the PK analysis in the phase 1 portion of this study, 10 mg/kg of uproleselan provided the highest levels of exposure that did not exceed the 14-day nonclinical safety limits, and this dose was selected for further testing in the phase 2 portion of the study.
R/R AML cohort
For the entire cohort of patients with R/R AML, the incidence of TEAEs, regardless of relationship to study drug, was similar across all uproleselan dose levels (supplemental Table 1). None of the 66 patients with R/R AML discontinued treatment because of an AE. As expected in patients with AML, all patients in this R/R cohort had evidence of grade 4 myelosuppression (thrombocytopenia, neutropenia, or anemia) during the study. For patients achieving a response (complete response [CR] or CR with incomplete count recovery [CRi]), the median time to count recovery (ANC ≥500/μL and platelets ≥50/μL) was 33.0 days (90% CI, 31.0-34.0). Table 2 shows the overall incidence of grade 3 or 4 TEAEs. Most of the TEAEs observed were typical of the background chemotherapy, with few TEAEs attributed to uproleselan (supplemental Table 2 provides an overview of TEAEs by dose). Apart from hematologic toxicities, the only grade 3 or 4 TEAEs reported for ≥10% of patients was sepsis (12% of patients). Gastrointestinal toxicities, including nausea, vomiting, diarrhea, and colitis, were mild, with grade 3 events occurring in <5% of patients. Hepatic and renal TEAEs were mostly grade 1 or 2, with grade 3 or 4 observed in 5% and 5% of patients, respectively. One patient (2%) died within 30 days of initiation of therapy; the 60-day mortality rate was 9% (n = 6). Grade 3 mucositis was reported in only 2% of patients. Other nonhematologic TEAEs were mild and generally judged to be unrelated to uproleselan.
Table 2.
Incidence (≥10%) of grade 3 or 4 TEAEs and TEAEs related to uproleselan
| TEAE | Patients, n (%) | |
|---|---|---|
| All TEAEs | TEAEs related to uproleselan | |
| R/R AML treated with MEC (n = 66) | ||
| Febrile neutropenia | 39 (59) | 11 (17) |
| Thrombocytopenia | 23 (35) | 7 (11) |
| Anemia | 17 (26) | 5 (8) |
| Platelet count decreased | 12 (18) | 4 (6) |
| Neutropenia | 11 (17) | 3 (5) |
| Sepsis | 8 (12) | 3 (5) |
| WBC count decreased | 7 (11) | 1 (2) |
| Hypophosphatemia | 6 (9) | 1 (2) |
| Neutrophil count decreased | 4 (6) | 1 (2) |
| Pneumonia | 3 (5) | 0 |
| Newly diagnosed AML treated with 7 + 3 (n = 25) | ||
| Febrile neutropenia | 22 (88) | 3 (12) |
| Anemia | 6 (24) | 2 (8) |
| Platelet count decreased | 6 (24) | 0 |
| Thrombocytopenia | 6 (24) | 3 (12) |
| WBC count decreased | 5 (20) | 1 (4) |
| Hypokalemia | 4 (16) | 0 |
| Neutrophil count decreased | 4 (16) | 0 |
| Pneumonia | 4 (16) | 2 (8) |
| Respiratory failure | 4 (16) | 0 |
| Hypophosphatemia | 3 (12) | 1 (4) |
| Neutropenia | 3 (12) | 1 (4) |
| Pancytopenia | 3 (12) | 0 |
| Pulmonary edema | 3 (12) | 1 (4) |
| Sepsis | 2 (8) | 0 |
MEC, mitoxantrone, etoposide, cytarabine; TEAE, Treatment emergent adverse events; WBC, white blood cell.
Newly diagnosed cohort
In the newly diagnosed cohort, the toxicities observed were exclusively related to the effects of underlying leukemia and the chemotherapy backbone (Table 2). As in patients with R/R disease, grade 4 myelosuppression was common. The median time to count recovery for neutrophils and platelets in responding patients was 32.0 days (90% CI, 28.0-32.0). Grade 3 or 4 nonhematologic TEAEs in this cohort occurring at a rate ≥10% included pneumonia (16%), hypokalemia (16%), respiratory failure (16%), pulmonary edema (12%), and hypophosphatemia (12%; supplemental Table 2 provides a summary of grade 3 or 4 TEAEs by dose). The 30- and 60-day all-cause mortality rates were 8% and 12%, respectively. An overview of TEAEs for patients in the newly diagnosed cohort is provided in supplemental Table 1.
Clinical response and survival
R/R cohort
Clinical outcomes are shown in Table 3. At the RP2D (n = 54), the CR/CRi rate was 41%, with most responses being CRs (35%). CR/CRi rates of 29% and 46% were observed in patients with primary refractory and relapsed disease, respectively. A CR/CRi rate of 52% was seen in patients with relapsed disease with 1 prior induction regimen, and the CR/CRi rate was 36% for patients treated with ≥2 prior regimens. Response rates by duration of initial response and cytogenetic risk are shown in Table 3. Among relapsed patients, a CR/CRi rate of 28% (7 of 25) was observed for patients with an initial CR duration of <12 months, compared with an 83% (10 of 12) response rate for patients with an initial CR duration of ≥12 months. Overall, 51% (34 of 66) of patients were previously treated with high doses of cytarabine (≥1 g/m2 dosing), either as a prior induction and/or as postremission therapy; the CR/CRi rate in these patients was 47%, similar to that in the entire R/R cohort.
Table 3.
Response rate summary for patients with R/R AML or newly diagnosed AML receiving uproleselan in combination with chemotherapy
| Outcome | Patients, n/N (%) | ||
|---|---|---|---|
| R/R (n = 66) | R/Ruproleselan at 10 mg/kg (n = 54) | Newly diagnosed patients age ≥60 y (n = 25) | |
| ORR (CR/CRi) | 26 (39) | 22 (41) | 18 (72) |
| Primary refractory | 8/22 (36) | 5/17 (29) | — |
| 1 induction regimen | 6/17 (35) | 4/13 (31) | — |
| ≥2 induction regimens | 2/5 (40) | 1/4 (25) | — |
| Relapsed | 18/44 (41) | 17/37 (46) | — |
| 1 induction regimen | 12/27 (44) | 12/23 (52) | — |
| ≥2 induction regimens | 6/17 (35) | 5/14 (36) | — |
| Duration of remission, mo | |||
| <6 | 4/22 (18) | 4/18 (22) | — |
| ≥6 | 14/22 (64) | 13/19 (78) | — |
| <12 | 8/32 (25) | 7/25 (28) | — |
| ≥12 | 10/12 (83) | 10/12 (83) | — |
| Prior HSCT | 5/12 (42) | 5/9 (56) | — |
| De novo (n = 12) | — | — | 9 (75) |
| Secondary (n = 13) | — | — | 9 (69) |
| CR | 22 (33) | 19 (35) | 13 (52) |
| CR/CRi/MLFS/PR | 32 (48) | 27 (50) | 20 (80) |
| MRD* | (n = 16) | (n = 13) | (n = 9) |
| Positive | 5 (31) | 4 (31) | 4 (44) |
| Negative† | 11 (69) | 9 (69) | 5 (56) |
| Proceeded to HSCT | 18 (27) | 17 (31) | 13 (52) |
| All-cause mortality, d | |||
| 30 | 1 (2) | 1 (2) | 2 (8) |
| 60 | 6 (9) | 5 (9) | 3 (12) |
CR, complete response; CRi, complete response with incomplete blood count recovery; d, days; HSCT, hematopoietic stem cell transplantation; MLFS, morphologic leukemia-free state; ORR, overall response rate; PR, partial response.
Evaluable patients.
MRD− bone marrow defined as ≤10−3 leukemic cells at end of induction by local assessment using multicolor flow cytometry, reverse transcription polymerase chain reaction, or next-generation sequencing.
Measurable residual disease (MRD), as assessed by multiparametric flow cytometry, was only evaluable for 16 patients in the R/R cohort. Of these, 11 patients (69%) were MRD− at the end of induction. After treatment with MEC plus the RP2D of uproleselan, 31% of patients (17 of 54) underwent allogeneic hematopoietic stem cell transplantation. Of the 22 patients achieving CR/CRi, 11 (50%) underwent transplantation.
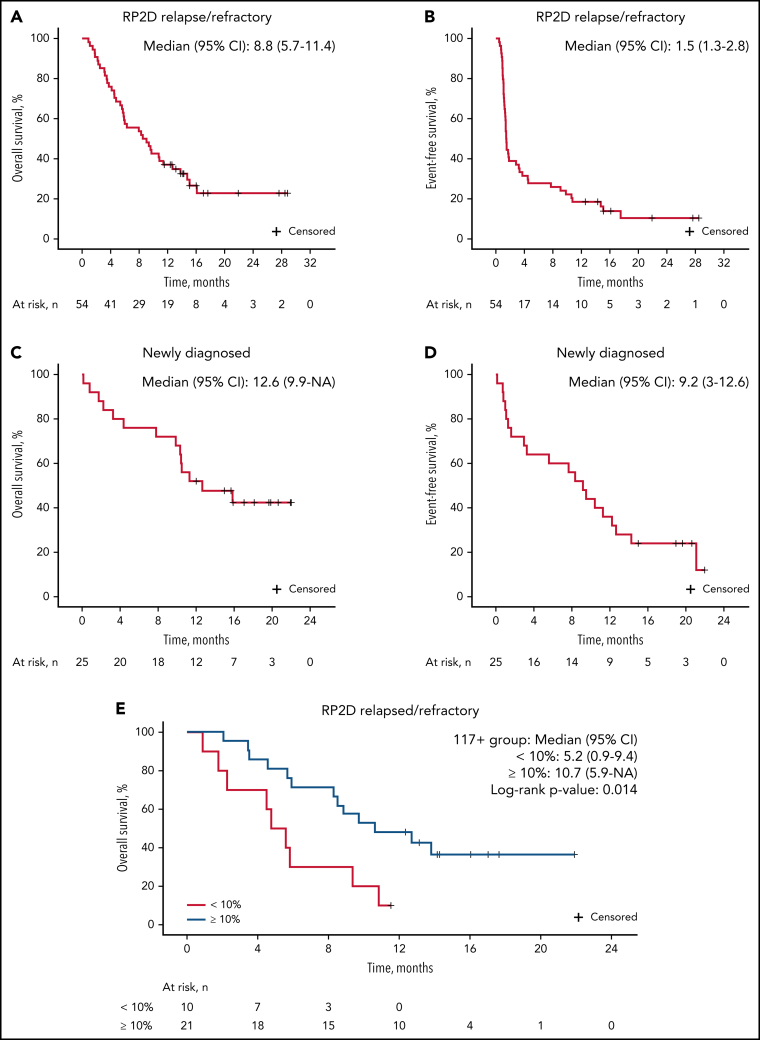
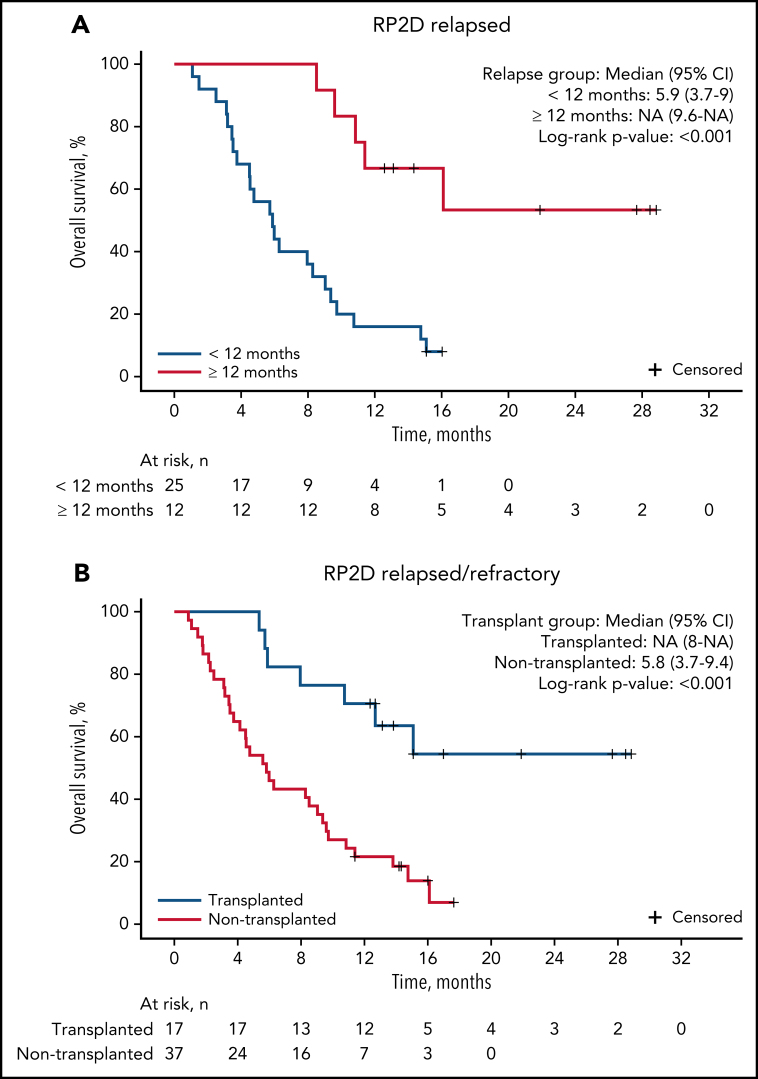
The median follow-up for patients on study was 9.7 months. For patients treated at the RP2D of uproleselan at 10 mg/kg, the median OS was 8.8 months (95% CI, 5.7-11.4; Figure 2A), and at 1 year, the probability of survival was 37.0%. The median event-free survival was 1.5 months (95% CI, 1.3-2.8; Figure 2B). The median duration of remission was 9.5 months (95% CI, 3.3 to not applicable; supplemental Figure 2A). For patients with relapsed disease with initial complete remission duration of <6 vs ≥6 months and <12 vs ≥12 months, duration of remission was not reached vs 13.8 months and 13.8 vs 16.4 months, respectively (supplemental Figures 4B and 3C). OS outcomes by duration of initial response (<12 vs ≥12 months and <6 vs ≥6 months), age, and ELN risk are shown in Figure 3A and supplemental Figures 4A, 3A, and 3B, respectively. Among patients who underwent transplantation after uproleselan at 10 mg/kg plus MEC, the median OS was not reached, and for those who did not undergo transplantation after uproleselan at 10 mg/kg plus MEC, the median survival was 5.8 months (Figure 3B). For patients previously exposed to high-dose cytarabine, the median survival was 9.2 months.
Figure 2.
OS and event-free survival with uproleselan at 10 mg/kg in combination with chemotherapy in patients with AML. OS (A) and event-free survival (B) among those with R/R disease; OS (C) and (D) event-free survival among those age ≥60 years with newly diagnosed disease; and OS (E) among patients with R/R disease according to E-selectin ligand expression ≥10% and <10%. AML, acute myeloid leukemia; NA, not applicable; RP2D, recommended phase 2 dose.
Figure 3.
OS among patients with AML with relapsed disease by initial CR duration and transplantation status after uproleselan in combination with chemotherapy. Patients with an initial CR duration of <12 months or ≥12 months (A), and patients who underwent transplantation after uproleselan at 10 mg/kg plus MEC and those who did not undergo transplantation after treatment (B). MEC, mitoxantrone/etoposide/cytarabine; NA, not applicable; RP2D, recommended phase 2 dose.
Newly diagnosed cohort
Clinical responses are summarized in Table 3. A CR/CRi was achieved by 18 (72%), with a majority (13 of 18) being CRs. Eleven (61%) of 18 responders achieved a CR/CRi with 1 cycle of induction. MRD analysis was evaluable for 9 of the responding patients, with 5 (56%) achieving an MRD− state at the end of induction. Nine (69%) of the newly diagnosed patients with secondary AML (n = 13) achieved a CR/CRi. Of 7 patients who had been previously treated with HMAs before the diagnosis of AML, 4 achieved a CR/CRi. Eleven patients (61%) achieving a CR/CRi underwent subsequent allogeneic transplantation after induction therapy with uproleselan and 7 + 3.
Among the 25 newly diagnosed patients who received uproleselan at 10 mg/kg in combination with 7 + 3, the median OS rate was 12.6 months (95% CI, 9.9 to not applicable; Figure 2C). At 12 months, the probability of survival was 52.0%. The event-free survival was 9.2 months (95% CI, 3-12.6; Figure 2D). The median duration of remission was 10.5 months (95% CI, 7.1 to not applicable; supplemental Figure 2B).
E-selectin ligand expression and impact on clinical outcome
E-selectin ligand expression on leukemic blasts and LSCs was available in 31 of 66 patients with R/R disease and 20 of 25 newly diagnosed patients. Expression of E-selectin ligand on LSCs was highly correlated with expression on leukemic blasts (supplemental Figure 5A). All evaluable patients had detectable levels of E-selectin ligand with expression in blasts and LSCs ranging from 0.41% to 96%.
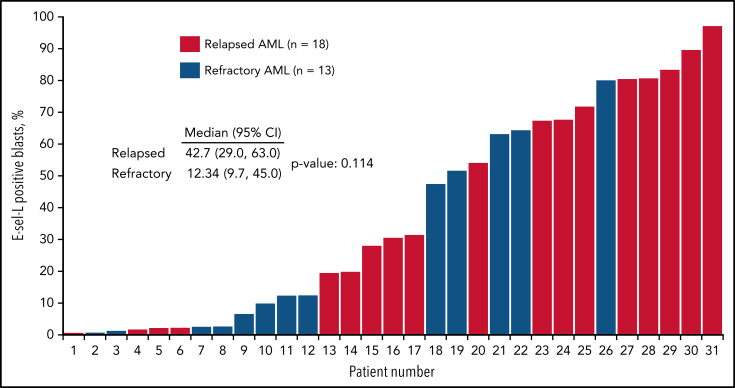
Of the 31 patient samples analyzed in the R/R cohort, 21 (67%) had detectable E-selectin ligand in ≥10% of blasts (high expressing), and 10 (32%) had levels <10% (low expressing; Figure 4). Expression levels ≥10% were more commonly detected in patients with relapsed disease (66%) and were less frequent in those with primary resistant disease. Adverse cytogenetics were more frequently observed in high-expressing patients (67% compared with 30% in low-expressing patients). CR/CRi rates for those with R/R AML were 38% (8 of 21) in high-expressing patients compared with 20% (2 of 10) in low-expressing patients. Of the 8 high-expressing patients achieving a CR/CRi, 6 underwent evaluation for MRD postinduction, with 67% (4 of 6) being MRD−. The median OS was 10.7 months in high-expressing patients compared with 5.2 months in low-expressing patients (Figure 2E).
Figure 4.
E-selectin ligand (E-sel-L) expression as proportion of blast population according to whether patient had R/R AML disease. AML, acute myeloid leukemia; E-sel-L, E-selectin ligand.
In samples from newly diagnosed patients, an E-selectin ligand expression of ≥10% of blasts was seen in 15 (75%) of 20 and <10% in 5 (25%) of 20 patients. Expression of E-selectin ligand on LSCs was also highly correlated with expression of leukemic blasts in newly diagnosed patients (supplemental Figure 5B). High-expressing patients were more likely to have secondary AML (73% [11 of 15]) and adverse cytogenetics (40% [6 of 15]) compared with low-expressing patients (40% [2 of 5] and 20% [1 of 5], respectively). The CR/CRi rate was 100% (5 of 5) in low-expressing patients and 67% (10 of 15) in high-expressing patients. The median OS was similar across all levels of E-selectin ligand expression in newly diagnosed patients.
Discussion
Survival rates for patients with R/R AML and older patients with newly diagnosed disease remain poor, primarily because of failure to eradicate LSCs.12 Emerging data have demonstrated that the bone marrow microenvironment contributes substantially to mechanisms of drug resistance in AML.13, 14 Bone marrow endothelial cell expression of E-selectin, resulting in binding to leukemic blasts, correlates with chemotherapy resistance.9 In this phase 1/2 trial, the E-selectin antagonist uproleselan added to both an intensive chemotherapy-based salvage regimen and a standard intensive frontline regimen was safe, well tolerated, and active.
Although uproleselan as a single agent has not been evaluated in patients with AML, nonclinical data from nonhuman primates and a study in healthy human volunteers demonstrated no significant organ toxicities (unpublished data). The MEC regimen has been well studied in the R/R AML population and is associated with a notable, albeit manageable, toxicity profile that includes myelosuppression and gastrointestinal (eg, mucositis and diarrhea) AEs.15, 16, 17 In this trial, the combination of uproleselan with MEC did not seem to delay count recovery or increase the incidence of serious AEs that would have been expected with MEC alone,18 and importantly, early mortality rates and severe comorbidities (eg, bacteremia and pneumonia) were not increased over those reported in this population when MEC is used alone. Similarly, the addition of uproleselan to the standard 7 + 3 regimen did not result in the increased toxicities that have been reported with 7 + 3 alone.19, 20
Of interest, the rate of severe mucositis occurring with uproleselan (grade 3 in just 2% of patients) was substantially lower than those historically reported with MEC.3 In addition to expression on bone marrow endothelium, E-selectin expression is increased at sites of inflammation, resulting in tethering, rolling, and extravasation of leukocytes, monocytes, macrophages, and neutrophils into inflamed tissue associated with chemotherapy toxicity.21 Thus, blockade of E-selectin outside the marrow may be associated with reduced inflammation. The observed low rate of severe mucositis in patients treated with MEC plus uproleselan suggests an additional beneficial effect of the on-target, off-leukemia effects of uproleselan.
Several investigational agents across multiple clinical trials have failed to improve outcomes for patients with R/R AML.4, 5 The observed 41% CR/CRi rate (a majority being CRs) and median survival rate of 8.8 months compare favorably with previously reported response rates in the 15% to 20% range and median OS rates of approximately 4 to 6 months.22, 23
Although only a small number of patients with an initial CR duration <6 months were treated, the response rates and median survival in this subgroup of patients were relatively low. Therefore, the benefit of the addition of uproleselan in this highly chemotherapy-resistant subpopulation of R/R AML remains unclear.
In this relatively small sample size of frontline patients, we noted several promising observations. Patients with AML that evolved from a prior hematologic disorder and who received prior treatment with an HMA are highly resistant to currently available induction chemotherapy regimens.24, 25, 26 Older patients who are eligible for intensive chemotherapy, a majority of whom have adverse-risk features, showed a high response rate with treatment with uproleselan plus 7 + 3 chemotherapy compared with 7 + 3 chemotherapy alone.27 This higher response rate was also seen in patients with secondary AML who had received prior therapy with HMAs. Preclinical data have demonstrated an upregulation of E-selectin ligand in cells preexposed to HMAs, suggesting a potential unique effect of uproleselan in this population.28 The ability of patients newly diagnosed with AML with high-risk features to undergo transplantation after initial response to therapy is highly desirable. In this study of patients age >60 years, an impressive 61% who achieved a CR/CRi underwent subsequent transplantation; the rate of transplantation in this study compares favorably to transplantation rates analyzed in recent reports.20
The distribution and clinical outcomes of patients with E-selectin ligand expression are of interest. Higher E-selectin expression was detected in patients with relapsed vs primary refractory disease and in patients with high-risk features (eg, secondary AML and adverse cytogenetics), both groups that are expected to have an enriched population of LSCs.29 A high expression of transferases (ST3Gal4 and FUT7) that allow surface glycoproteins on leukemic blasts and LSCs to bind to E-selectin correlates with a high relapse rate and shorter survival in children with AML who are treated with induction chemotherapy.30 Furthermore, cellular adhesion–mediated drug resistance, driven in part by E-selectin/E-selectin ligand and CXCR4/CXCL12 interactions between LSCs and stromal/endothelial cells in the osteoblastic and vascular bone marrow niches, has been hypothesized to be a crucial component allowing LSCs to survive cytotoxic therapies.31, 32 Although the data set was relatively small in this R/R AML cohort, the median survival rate of 10.7 months in patients with expression levels ≥10% represents a major improvement over historical control data. Similarly, the 67% response rate in patients with newly diagnosed AML with expression levels ≥10% is noteworthy, given the high incidence of secondary AML and adverse cytogenetics in these patients. Taken together, these data suggest that E-selectin ligand expression contributes to chemotherapy resistance, which may be overcome with uproleselan and could be an important predictor of response and survival.
In summary, the results from this phase 1/2 clinical trial support the biologic and clinical rationales for targeting E-selectin and support the need for a confirmatory randomized controlled trial to further evaluate the benefits of adding uproleselan to salvage chemotherapy regimens in patients with R/R disease and anthracycline-based treatments in older patients with AML. Randomized trials are ongoing in both populations.
Acknowledgments
The authors thank all study investigators, coordinators, and pharmacists; Rho, Inc. (Durham, NC), for statistical support; Novella Clinical (now IQVIA Biotech) (Morrisville, NC) for trial monitoring and operations; additional members of the GlycoMimetics staff; and the patients who participated in the study. Medical writing assistance (funded by GlycoMimetics) was provided by Lamara D. Shrode, JB Ashtin, who, on behalf of the authors, assisted in writing the first draft, implemented author revisions throughout the editorial process, and prepared the manuscript for submission.
The design, conduct, analysis, and financial support of the clinical trial were provided by GlycoMimetics.
GlycoMimetics participated in the interpretation of data, reviewed the manuscript, and approved the submission of the manuscript for possible publication.
Footnotes
Presented in abstract form at the 60th Annual Meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018.
Original data will be available for 2 years, beginning 6 months after approval of the study drug for use in patients with AML. Send data access proposals to clinicaltrials@glycomimetics.com. The study protocol is included as an online supplement available with the online version of this article.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Building on compelling preclinical research, DeAngelo et al report the results of a phase 1/2 trial of uproleselan, an E-selectin inhibitor, in combination with 2 different standard chemotherapy regimens in patients with acute myeloid leukemia. Their data suggest antileukemic activity through targeting of the bone marrow microenvironment and form the basis for a randomized trial.
Authorship
Contribution: H.M.T., J.L.M., E.J.F., D.J.D., B.A.J., and P.M. designed the trial and collected and analyzed the data; D.J.D., B.A.J., J.L.L., D.L.B., A.S.A., P.M., M.E.O., and P.S.B. had access to the raw data for their sites; and all authors interpreted the data, critically reviewed the manuscript, and provided final approval for submission. All authors agree to be accountable for all aspects of the work, ensuring the accuracy and integrity of the publication.
Conflict-of-interest disclosure: D.J.D. has received funding/grant support from AbbVie, Blueprint Medicines, GlycoMimetics, and Novartis and honoraria or consulting fees from Amgen, Agios, Astella, Bristol-Myers Squibb, Blueprint Medicines, Incyte, Jazz, Novartis, Pfizer, Servier, and Takeda. B.A.J. has received funding/grant support (to his institution) from 47, AbbVie, Accelerated Medical Diagnostics, Amgen, AROG, Celgene, Daiichi Sankyo, Esanex, F. Hoffmann-La Roche, Forma, Genentech/Roche, GlycoMimetics, Hanmi, Incyte, Jazz, LP Therapeutics, Pfizer, Pharmacyclics, and Sigma-Tau; travel reimbursement from AbbVie and Amgen; and honoraria or consulting fees from AbbVie, Amgen, Celgene, Genentech, GlycoMimetics, Jazz, Takeda, Tolero, and Treadwell. J.L.L. has received honoraria or consulting fees from AbbVie and Onconova. D.L.B. has received funding/grant support from GlycoMimetics. A.S.A. has received funding/grant support from Amgen and Pfizer and honoraria or consulting fees from GlycoMimetics, Novartis, and Pfizer. P.M. has received funding/grant support from GlycoMimetics and honoraria, consulting fees, or speaker fees from AbbVie, Astellas, AstraZeneca, Celgene, Gilead, Janssen, Jazz, Novartis, Pfizer, and Roche. M.E.O.B. has received funding/grant support from Bristol-Myers Squibb, Celgene, GlycoMimetics, Janssen, ONK Therapeutics; has served on advisory boards for AbbVie, Janssen, and ONK Therapeutics; and reports equity ownership in ONK Therapeutics. P.S.B. has received institutional grant support from AbbVie, Bristol-Myers Squibb, Cardiff Oncology, GlycoMimetics, JW Pharmaceutical, Novartis, Pfizer, and SecuraBio. J.LM., H.M.T., and E.J.F. are full-time employees of GlycoMimetics and may hold Glyco Mimetics stock or stock options.
The current affiliation for P.S.B. is Hematology/Oncology Division in the Department of Medicine, School of Medicine, University of California Irvine, Irvine, CA.
Supplementary Material
REFERENCES
- 1.Estey EH. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol. 2018;93(10):1267–1291. doi: 10.1002/ajh.25214. [DOI] [PubMed] [Google Scholar]
- 2.Faderl S, Wetzler M, Rizzieri D, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J Clin Oncol. 2012;30(20):2492–2499. doi: 10.1200/JCO.2011.37.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman EJ, Brandwein J, Stone R, et al. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol. 2005;23(18):4110–4116. doi: 10.1200/JCO.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 4.Ravandi F, Ritchie EK, Sayar H, et al. Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): a randomised, controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2015;16(9):1025–1036. doi: 10.1016/S1470-2045(15)00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roboz GJ, Rosenblat T, Arellano M, et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol. 2014;32(18):1919–1926. doi: 10.1200/JCO.2013.52.8562. [DOI] [PubMed] [Google Scholar]
- 6.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 7.Kunkel EJ, Ley K. Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ Res. 1996;79(6):1196–1204. doi: 10.1161/01.res.79.6.1196. [DOI] [PubMed] [Google Scholar]
- 8.Gruszka AM, Valli D, Alcalay M. Wnt signalling in acute myeloid leukaemia. Cells. 2019;8(11):1403. doi: 10.3390/cells8111403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbier V, Erbani J, Fiveash C, et al. Endothelial E-selectin inhibition improves acute myeloid leukaemia therapy by disrupting vascular niche-mediated chemoresistance. Nat Commun. 2020;11(1):2042. doi: 10.1038/s41467-020-15817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death—a new approach to cancer therapy. J Clin Invest. 2005;115(10):2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheson BD, Bennett JM, Kopecky KJ, et al. International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia [published correction appears in J Clin Oncol. 2004;22(3):576] J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 12.Pollyea DA, Jordan CT. Therapeutic targeting of acute myeloid leukemia stem cells. Blood. 2017;129(12):1627–1635. doi: 10.1182/blood-2016-10-696039. [DOI] [PubMed] [Google Scholar]
- 13.Rashidi A, Uy GL. Targeting the microenvironment in acute myeloid leukemia. Curr Hematol Malig Rep. 2015;10(2):126–131. doi: 10.1007/s11899-015-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabe Y, Konopleva M. Role of microenvironment in resistance to therapy in AML. Curr Hematol Malig Rep. 2015;10(2):96–103. doi: 10.1007/s11899-015-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg PL, Lee SJ, Advani R, et al. Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome: a phase III trial (E2995) J Clin Oncol. 2004;22(6):1078–1086. doi: 10.1200/JCO.2004.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price SL, Lancet JE, George TJ, et al. Salvage chemotherapy regimens for acute myeloid leukemia: is one better? Efficacy comparison between CLAG and MEC regimens. Leuk Res. 2011;35(3):301–304. doi: 10.1016/j.leukres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Trifilio SM, Rademaker AW, Newman D, et al. Mitoxantrone and etoposide with or without intermediate dose cytarabine for the treatment of primary induction failure or relapsed acute myeloid leukemia. Leuk Res. 2012;36(4):394–396. doi: 10.1016/j.leukres.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Halpern AB, Othus M, Huebner EM, et al. Phase 1/2 trial of GCLAM with dose-escalated mitoxantrone for newly diagnosed AML or other high-grade myeloid neoplasms. Leukemia. 2018;32(11):2352–2362. doi: 10.1038/s41375-018-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert J, Pautas C, Terré C, et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica. 2019;104(1):113–119. doi: 10.3324/haematol.2018.188888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684–2692. doi: 10.1200/JCO.2017.77.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chase SD, Magnani JL, Simon SI. E-selectin ligands as mechanosensitive receptors on neutrophils in health and disease. Ann Biomed Eng. 2012;40(4):849–859. doi: 10.1007/s10439-011-0507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bose P, Vachhani P, Cortes JE. Treatment of relapsed/refractory acute myeloid leukemia. Curr Treat Options Oncol. 2017;18(3):17. doi: 10.1007/s11864-017-0456-2. [DOI] [PubMed] [Google Scholar]
- 23.Sasine JP, Schiller GJ. Emerging strategies for high-risk and relapsed/refractory acute myeloid leukemia: novel agents and approaches currently in clinical trials. Blood Rev. 2015;29(1):1–9. doi: 10.1016/j.blre.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Ball B, Komrokji RS, Adès L, et al. Evaluation of induction chemotherapies after hypomethylating agent failure in myelodysplastic syndromes and acute myeloid leukemia. Blood Adv. 2018;2(16):2063–2071. doi: 10.1182/bloodadvances.2018015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bello C, Yu D, Komrokji RS, et al. Outcomes after induction chemotherapy in patients with acute myeloid leukemia arising from myelodysplastic syndrome. Cancer. 2011;117(7):1463–1469. doi: 10.1002/cncr.25598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaglal MV, Duong VH, Bello CM, et al. Cladribine, cytarabine, filgrastim, and mitoxantrone (CLAG-M) compared to standard induction in acute myeloid leukemia from myelodysplastic syndrome after azanucleoside failure. Leuk Res. 2014;38(4):443–446. doi: 10.1016/j.leukres.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Lancet JE, Cortes JE, Hogge DE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123(21):3239–3246. doi: 10.1182/blood-2013-12-540971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fogler WE, Smith TAG, Lee J-W, Magnani JL. Glycomimetic antagonist of E-selectin, GMI-1271, enhances therapeutic activity of the hypomethylating agent, 5-azacitidine, in the KG1 model of AML. Blood. 2017;130:5065. suppl 1): [Google Scholar]
- 29.Chen J, Kao YR, Sun D, et al. Myelodysplastic syndrome progression to acute myeloid leukemia at the stem cell level [published correction appears in Nat Med. 2019;25(3):529.] Nat Med. 2019;25(1):103–110. doi: 10.1038/s41591-018-0267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonti AR, Pardo L, Alonzo TA, et al. Transcriptome profiling of glycosylation genes defines correlation with E-selectin ligand expression and clinical outcome in AML. Blood. 2019;134:3772. suppl 1): [Google Scholar]
- 31.Becker PS, Kopecky KJ, Wilks AN, et al. Very late antigen-4 function of myeloblasts correlates with improved overall survival for patients with acute myeloid leukemia. Blood. 2009;113(4):866–874. doi: 10.1182/blood-2007-12-124818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Natoni A, Smith TAG, Keane N, et al. E-selectin ligands recognised by HECA452 induce drug resistance in myeloma, which is overcome by the E-selectin antagonist, GMI-1271. Leukemia. 2017;31(12):2642–2651. doi: 10.1038/leu.2017.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.