Evaluation of reaction condition for pyrrolidine α-arylationa.
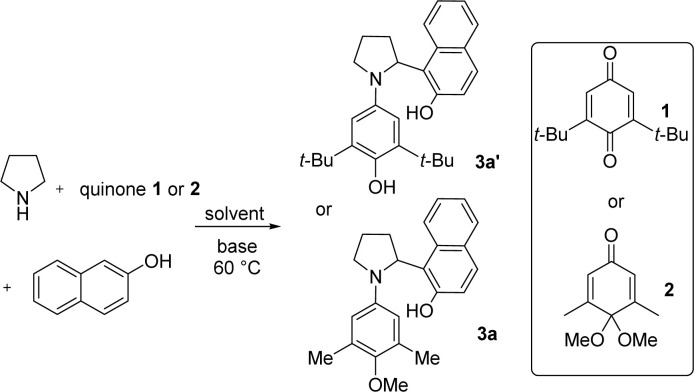
| ||||
|---|---|---|---|---|
| Entry | Quinone | Base (equiv.) | Solvent | Yield |
| 1 | 1 | TFE | 3a′ (trace) | |
| 2 | 1 | i-PrOH | 3a′ (trace) | |
| 3 | 1 | PhCH3 | 3a′ (trace) | |
| 4 | 1 | DBU (0.1) | PhCH3 | 3a′ (trace) |
| 5 | 1 | DABCO (0.1) | PhCH3 | 3a′ (30%) |
| 6 | 2 | DABCO (0.1) | PhCH3 | 3a (59%) |
| 7 | 2 | DABCO (0.2) | PhCH3 | 3a (84%) |
| 8 | 2 | DABCO (0.5) | PhCH3 | 3a (64%) |
| 9 | 2 | DABCO (0.2) | TFE | 3a (trace) |
| 10 | 2 | DABCO (0.2) | Bezene | 3a (84%) |
| 11 | 2 | DABCO (0.2) | p-Xylene | 3a (80%) |
| 12 | 2 | DABCO (0.2) | MeOH | 3a (25%) |
| 13 | 2 | DABCO (0.2) | EtOH | 3a (37%) |
| 14 | 2 | DABCO (0.2) | CH3CN | 3a (30%) |
| 15b | 2 | DABCO (0.2) | PhCH3 | 3a (91%) |
Reactions were performed with pyrrolidine (0.33 mmol), β-naphthol (0.45 mmol), and quinone monoacetal 2 (0.3 mmol) at 60 °C in solvent (conc. = 0.3 M), isolated yield.
Reaction was performed in toluene (conc. = 0.5 M).
