Abstract
Molecular mechanisms of the interaction between opioidergic and dopaminergic processing during pain-related experiences in the human brain are still incompletely understood. This is partially due to the invasive nature of the available techniques to visualize and measure metabolic activity. Positron Emission Tomography (PET) radioligand studies using radioactive substances are still the only available modality to date that allows for the investigation of the molecular mechanisms in the human brain. The most commonly studied PET radiotracers are [11C]-carfentanil (CFN) and [11C]- or [18F]-diprenorphine (DPN), which bind to opioid receptors, and [11C]-raclopride (RAC) and [18F]-fallypride (FAL) tracers, which bind to dopamine receptors. The current meta-analysis examines pain-related studies that used aforementioned opioid and dopamine radioligands in an effort to consolidate the available data into the most likely activated regions. Our primary goal was to identify regions of shared opioid/dopamine neurotransmission during pain-related experiences using within-subject approach. Seed-based d Mapping (SDM) analysis of previously published voxel coordinate data showed that opioidergic activations were strongest in the bilateral caudate, thalamus, right putamen, cingulate gyrus, midbrain, inferior frontal gyrus, and left superior temporal gyrus. The dopaminergic studies showed that the bilateral caudate, thalamus, right putamen, cingulate gyrus, and left putamen had the highest activations. We were able to see a clear overlap between opioid and dopamine activations in a majority of the regions during pain-related experiences, though there were some unique areas of dopaminergic activation such as the left putamen. Regions unique to opioidergic activation included the midbrain, inferior frontal gyrus, and left superior temporal gyrus. Here we provide initial evidence for the functional overlap between opioidergic and dopaminergic processing during aversive states in humans.
1. Introduction
Pain conditions are widespread, affecting around 20% of the US population, and are often treated with opioid medications (Dahlhamer et al., 2018). However, recently there has been a push to reduce opioid use, as such medications often lead to addiction (Cowan et al., 2003; Fishbain et al., 2008). Notably, consumption of exogenous opioids lead to changes within endogenous opioid and dopamine neurotransmission (Kosten and George, 2002), both of which play fundamental roles in pain processing, pain modulation, and pain relief (Fields, 2018). Investigating whether dopaminergic and opioidergic activity are released in the same regions during similar conditions may enlighten us on how it is they work together to create and process the perception of pain/relief.
Functional MRI studies have allowed us to observe how various brain regions are influenced by experimental and clinical pain conditions. Among these, the insular cortex is activated in a wide variety of pain paradigms as well as in emotional and interoceptive aspects of pain (Jensen et al., 2016). The brain stem and anterior cingulate cortex provide connectivity among brain areas related to the subjective perception and modulation of pain (Ploner et al., 2010). The ventral striatal area, specifically the nucleus accumbens (NAc) is part of the reward system that modulates dopamine signaling in response to a pain stimulus, the activation of this area occurs with both acute pain and pain relief (Baliki et al., 2010; Becerra et al., 2013). The PFC has been widely implicated in the neuromodulation of pain through its connections with areas such as the cerebral neocortex, hippocampus, periaqueductal grey (PAG), thalamus, amygdala, and basal nuclei (Ong et al., 2019).
Nevertheless, the only method by which to measure neurotransmission in the human brain is still Positron Emission Tomography (PET). The endogenous opioidergic system is normally assessed using [11C]-carfentanil (CFN) and [11C]- or [18F]-diprenorphine (DPN) as radiotracers. CFN is a selective agonist at the μ-opioid receptor (MOR), which is thought to preferentially bind to the μ1 receptor sub-type (Eriksson and Antoni, 2015), whereas DPN is a weak partial agonist with equal affinity for the MOR, κ-opioid receptor (KOR), and δ-opioid receptor (DOR). The endogenous dopaminergic system is normally assessed by [11C]-raclopride (RAC) and [18F]-fallypride (FAL) tracers, which are selective antagonists on D2/D3 dopamine receptors. Information about both basal levels of receptor availability and changes in availability caused by alterations in endogenous dopamine and opioid concentrations can be evaluated, as these radioligands are sensitive to endogenous opioid and dopamine levels. PET radioligand studies have provided valuable information on the idiosyncratic concentrations and distributions of such neurochemicals across the brain, and researchers have used these data to better understand aversive processing related to physical and emotional pain (e.g. (Hsu et al., 2013; Zubieta et al., 2001), as well as addiction processes associated with opioids (Greenwald et al., 2003) and dopamine (Volkow et al., 2009). Notably, as demonstrated by prior animal studies (Benarroch, 2012), there is anatomical overlap of neurotransmitter systems. Studies demonstrating such overlap of these neurochemicals in the human brain are limited (Goldman-Rakic et al., 1990) and one of the major limitations of current PET technology is that it only allows for the imaging of a single radiotracer at a time. Recent research has provided insight into the fact that endogenous opioids are released in the ventral striatum, insula, anterior cingulate cortex (ACC), prefrontal cortex (PFC), and brain stem during aversive experiences, though knowing whether dopamine is also released in many of the same regions during similar aversive conditions may demonstrate that the two neurotransmitter systems might be working together to create and process the perception of pain/relief. Thus, an increased understanding of the brain regions where these neurotransmitters co-localize in the human brain may further elucidate pain and aversive processing. The present dual meta-analysis specifically aims to create a map of the opioid and dopamine neurotransmitter systems in the brain through aversive (“hurt”) conditions.
1.1. Neural Correlates of Opioid Receptors
The endogenous opioid system consists of 3 families of opioid peptides: β-endorphin, enkephalins, and dynorphins, and 3 families of receptors: μ (MOR), δ (DOR), and κ (KOR) (reviewed in detail elsewhere e.g. (Benarroch, 2012). Though endogenous opioids can be found extensively throughout the central and peripheral nervous system, they are particularly concentrated in circuits involved in pain modulation, pain relief, responses to stress, and autonomic control. Some of the areas with the highest opioidergic receptor concentration are the cerebral cortex, brainstem, thalamus, striatum, hypothalamus, hippocampus, and dorsal horn (Benarroch, 2012). Radioligand studies of opioidergic receptors included in this meta-analysis have reported opioid activations in a number of cortical and subcortical regions, including frontal cortices (Dougherty et al., 2008; Jones et al., 1999; Klega et al., 2010; Maarrawi et al., 2013; Mueller et al., 2010; Wey et al., 2014; Willoch et al., 2004), insula (Brown et al., 2015; Dougherty et al., 2008; Jones et al., 1999; Mueller et al., 2010; Wey et al., 2014; Willoch et al., 2004), anterior cingulate cortex (Dougherty et al., 2008; Jones et al., 1999; Maarrawi et al., 2013; Mueller et al., 2010; Sprenger et al., 2006; Wey et al., 2014; Willoch et al., 2004), thalamus (Brown et al., 2015; Dougherty et al., 2008; Jones et al., 1999; Wey et al., 2014; Willoch et al., 2004) and putamen (Brown et al., 2015; Jones et al., 1999; Wey et al., 2014).
1.2. Neural Correlates of Dopamine Receptors
Dopamine is widely involved in circuits encoding reward, aversion, salience, uncertainty, novelty (Bromberg-Martin et al., 2010), and pain modulation (Wood, 2008). The midbrain houses some of the areas with the highest dopaminergic concentrations, namely the Ventral Tegmental Area (VTA) and Substantia Nigra (SN), as well as projections that lead to the dorsal striatum, nucleus accumbens, amygdala, hippocampus, and prefrontal cortex (PFC). Radioligand studies of dopaminergic receptors have reported activations in a number of cortical and subcortical regions, including bilateral putamen (Berman et al., 2013; Scott et al., 2008; Wood et al., 2007), left putamen (Martikainen et al., 2015), left caudate (Berman et al., 2013; Martikainen et al., 2015; Scott et al., 2007; Scott et al., 2008; Wood et al., 2007), right caudate nucleus (Martikainen et al., 2015; Scott et al., 2008; Wood et al., 2007), right nucleus accumbens (Martikainen et al., 2015; Pecina et al., 2015; Scott et al., 2008), left nucleus accumbens (Scott et al., 2008), and globus pallidus (Wood et al., 2007).
1.3. Interaction between opioidergic and dopaminergic neurotransmission
In many regions, opioids are co-expressed with other neurotransmitters (Benarroch, 2012). Specifically, opioid neurotransmitter systems interact with dopamine not only in the midbrain but also in projection areas such as the striatum, a key area implicated in pain relief (Fields, 2018). In addition to its established role in the reward system, many studies have suggested that the NAc also serves as a meeting point for multiple components of pain processing and analgesia. Some of the regions connected by the NAc include the ACC, PFC, thalamus, amygdala, somatosensory cortex, and the spinal cord, which span the reward and pain systems (Harris and Peng, 2020). Striatal medium spiny neurons express both dopamine and opioid receptors (Ambrose et al., 2004; Pollard et al., 1977). Blocking striatal opioid receptors leads to attenuated amphetamine-induced locomotion and impulsivity (Gonzalez-Nicolini et al., 2003; Wiskerke et al., 2011), whereas dopamine D2 receptor (DRD2) blockade inhibits the rewarding effects of morphine in opiate dependent rats (Laviolette et al., 2002). Likewise, activating μ-opioid receptors (MOR) modulates the mesolimbic dopamine system. As shown in rats, morphine modulates the release of dopamine by disinhibition through GABAergic interneurons in the midbrain ventral tegmental area (VTA) (Jalabert et al., 2011). In humans, alfentanil triggers dopamine release in the striatum (Hagelberg et al., 2002). Likewise, activating D2/D3 receptors modulate mesolimbic opioid system. As shown in several studies in humans, amphetamine releases endogenous opioids in the striatum, as well as in insular and anterior cingulate cortices (Colasanti et al., 2012; Mick et al., 2014), confirming the interdependence of the two systems. To date only a few studies have examined the overlap between opioidergic and dopaminergic receptor binding in the same individual, though there is data showing overlap in the ventral striatum and dorsal caudate nucleus (Tuominen et al., 2015).
From current literature it is evident that there is high variability across PET radioligand studies regarding the foci of activations. These inconsistencies may be attributed to several factors including, but not limited to, demographic characteristics of the sample such as age and sex, the presence of comorbid disorders and/or childhood adversity, differences in protocols and processing, and statistical analyses. Here we applied seed-based d mapping meta-analyses (Radua et al., 2012) that have been established as a standard tool for identifying coordinate- based convergence of voxel-based imaging data. This analysis was conducted separately for the opioidergic radioligands and for the dopaminergic radioligands and enabled us to explore overlap between the two neurotransmitter systems in aversive experiences.
2. Methods:
2.1. Article Selection
We conducted a literature search for radioligand PET studies that were published between June 1999 and March 2022 using several sources including PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) and Embase (https://www.embase.com) to find reports with the radioligands of interest such as “carfentanil”, “diprenorphine”, “raclopride”, “fallypride”. The study Flow Chart is depicted in Figure 1. The final article search was conducted on March 2022 and yielded 393 PET imaging studies using the radioligands of interest to be considered for further review. The studies included in our analysis mapped brain regions associated with opioidergic and dopaminergic receptors using CFN, DPN, RAC and FAL radioligands. We investigated general opioid receptors (MOR, DOR, and KOR) via diprenorphine (DPN) while selective radioligands like carfentanil (CAR) was used to study MOR. Both fallypride (FAL) and raclopride (RAC) were used to study dopamine type-2 and type-3 (D2/D3) receptors. D1-receptor specific radioligands were considered for additional data of the dopaminergic system, though only two radioligands are used for human subjects, [11C]SCH 23390 and [11C]NNC-112. After conduction an article search, it was determined that there were no PET studies investigating pain conditions using these radioligands.
Fig. 1.
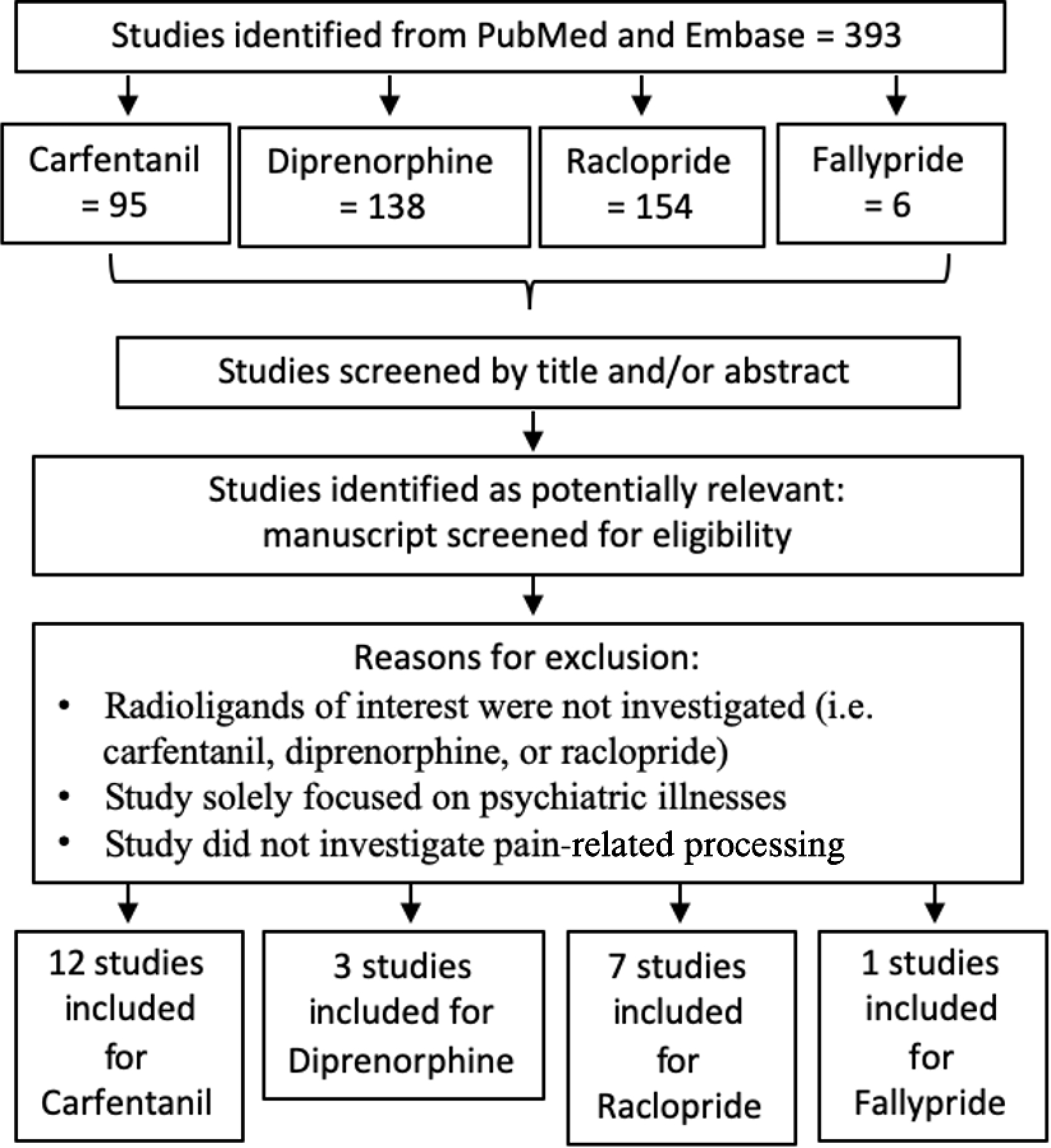
Flow chart of search strategy and study selectin for meta-analyes Study search and screening procedures repeated for Pub med and Science Direct.
All studies of pain-related aversive processing that provided voxel-based coordinates of the observed peak activations were included. Due to the variable nature of PET radioligand studies, i.e., examining acute and chronic pain conditions as well as correlational changes, we limited our inclusion to studies only reporting within-group observations, i.e., only those studies that reported changes in endogenous dopaminergic or opioidergic transmission as a result of experimental pain/aversion or endogenous chronic pain manipulation (painful/aversive state vs. non-pain state). Studies reporting between-group observations (i.e., changes in the endogenous neurotransmission between healthy subjects and individuals with chronic pain) were excluded from the meta-analyses.
2.2. Inclusion/exclusion criteria for activation foci
Significant peak activation coordinates and corresponding intensity values for each of the articles in Table 1 were extracted for differences in experimental conditions. If studies included two separate control conditions, only foci from one of the within-subject comparisons were used to avoid using foci from the same participant twice. In these cases, the selected contrast compared a painful or unpleasant condition with a non-painful condition. If a study conducted a whole-brain and a region of interest (ROI)-based analysis, coordinates from both analyses were included separately, provided that the ROIs were not reported in the whole-brain results.
Table 1:
Characteristics of studies included in the meta-analysis
| Study | Sample | Condition | Stimulation | Method of stimulation | Sex (M,F) | N | Age | Type | Significance |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Opioid | |||||||||
|
| |||||||||
| Carfentanil | |||||||||
|
| |||||||||
| Prossin, 2015 | Healthy controls | Pain Increase | Masseter muscle pain | Hypertonic Saline | 12, 22 | 34 | 22±11 | WBA | p<0.001 |
| Zubieta, 2001 | Healthy controls | Pain Increase | Masseter muscle pain | Hypertonic Saline | 13,7 | 20 | 24±2 | WB | p<0.05 |
| Scott, 2007 | Healthy controls | Pain Increase | Masseter muscle pain | Hypertonic Saline | 14,0 | 15 | 27±5 | WBA & ROI | p < 0.01 |
| Wager, 2007 | Healthy controls | Pain Increase | Thermal Pain | Thermode | n/a | 15 | 25±5 | ROI | p < 0.001 |
| Scott, 2008 | Healthy controls | Pain Increase | Masseter muscle pain | Hypertonic Saline | 9, 11 | 20 | 24±3 | WBA | p < 0.0001 |
| DaSilva, 2014 | Migraine | Pain Increase | Headache pain | During headache | 3,4 | 12 | n/a | WBA | n/a |
| Nascimento, 2014 | Migraine | Pain Increase | Thermal Pain | Thermode | 3, 3 | 6 | 26 | WBA | p < 0.0001 |
| Pecina, 2015 | Healthy controls | Pain Increase | Masseter muscle pain | Hypertonic Saline | 21, 29 | 50 | 26±0.7 | WBA | p < 0.0001 |
| Zubieta, 2003 | Healthy controls | Pain Increae | Sad mood induction | Autobiographical memory | 0, 14 | 14 | 36±9 | WBA | P < 0.001 |
| Hsu, 2013 | Healthy controls | Pain Increase | Rejection Pain | Social feedback task | 5, 13 | 18 | 32±12 | VOI | p < 0.05; p < 0.01 |
| Harris, 2009 | Fibromyalgia | Pain Releif | Fibromyalgia pain | Accupuncture | 0, 20 | 20 | 44.3±13.6 | WBA & ROI | P < 0.001 |
| Pecina, 2013 | Healthy controls | Pain Relief | Masseter muscle pain | Placebo | 19, 18 | 37 | 30±10 | WBA & ROI | p < 0.001; p < 0.05 |
| Diprenorphine | |||||||||
|
| |||||||||
| Maarrawi, 2007 | Neuropathic pain | Pain Increae | Chronic pain | Contralateral pain | 4, 4 | 8 | 55.2±11 | ROI | p < 0.0005 |
| Wey, 2014 | Healthy controls | Pain Increase | Pressure pain | Pressure cuff | 4, 4 | 8 | 24 | ROI | p < 0.05 |
| Jones, 1999 | Trigeminal Neralgia | Pain relief | Neuralgia pain | Surgery | 6,0 | 6 | 61.5 ± 19.5 | ROI | p < 0.01 |
| Dopamine | |||||||||
| Radopride | |||||||||
|
| |||||||||
| Berman, 2013 | Writer’s cramp | Pain Increae | Writer’s cramp pain | Tapping Task | 10, 5 | 15 | 52.41 ± 9 | WBA & ROI | p < 0.05 |
| Martikainen, 2015 | CNBP patients | Pain Increae | Masseter muscle pain | Hypertonc saline | 18, 14 | 16 | 35 ± 11 | WBA & ROI | p < 0.05; p < 0.005 |
| Pecina, 2015 | Healthy controls | Pain Increae | Masseter muscle pain | Hypertonc saline | 21, 29 | 50 | 26±0.7 | WBA | p<0.05; p < 0.0001 |
| Scott, 2006 | Healthy controls | Pain Increae | Masseter muscle pain | Hypertonc saline | 18, 7 | 25 | 27±5 | WBA | p<0.0001; p<0.01 |
| Scott, 2007 | Healthy controls | Pain Increae | Masseter muscle pain | Hypertonc saline | 14, 0 | 14 | 27±5 | ROI | P < 0.01 |
| Scott, 2008 | Healthy controls | Pain Increae | Masseter muscle pain | Hypertonc saline | 9, 11 | 20 | 24±3 | WBA & ROI | p = 0.05, p<0.0001 |
| Wood, 2007 | FM patients | Pain Increae | Tibialis muscle pain | Hypertonc saline | 0, 22 | 11 | n/a | ROI | p < 0.05 |
| Fally pride | |||||||||
|
| |||||||||
| Jarcho, 2015 | Healthy controls | Pain Increase | Thermal Pain | Thermode | 0, 15 | 15 | 24±3.11 | WBA | p = 0.001 |
FM = Fibromyalgia; WBA =Whole brain analysis; VOI = Volume of interest; ROI = Region of interest; CNBP = Chronic non-neuropathic back pain
Twenty-three radioligand studies (Table 1) were included in the current investigation based on the inclusion and exclusion criteria, and produced a total of 451 subjects from which peak voxels and clusters were extracted and transformed into Montreal Neurological Institute (MNI) space if they were provided in Talairach space. Of the 393 articles found, 368 were excluded for the following reasons: 1) Radioligands of interest were not investigated (i.e. CFN, DPN, RAC or FAL), 2) study solely focused on how neurological disorders affect the neurotransmitter of interest (e.g. Parkinson’s Disease), 3) study did not investigate aversive processing; 4) study provided only correlational relationship with the baseline neurotransmitter release; and 5) studies only reported on the between-group comparisons between healthy controls and individuals with chronic pain without within-subject experimental manipulation.
2.3. Meta-analysis
We conducted a coordinate-based random-effects meta-analysis using Sdm-Psi software version 6.21 (https://www.sdmproject.com/). Coordinates reported in Talairach space were converted to MNI space using the “convert peaks” tool provided by Sdm-Psi. We also gathered the peak intensity for each coordinate in the form of t-scores, otherwise they were converted from z-scores or p-values into t-scores using the “Convert peaks” feature of Sdm-Psi. Twelve studies were identified that examined mu-opioid receptors using CFN and 3 studies were identified that assayed all opioid receptors using DPN; these 15 studies were included in the opioidergic meta-analysis. Seven studies were used to examine D2/D3 receptors using RAC and 1 study used FAL radioligand; these 8 studies were included in the dopaminergic meta-analysis.
An anisotropic effect-size-based seed-based d mapping (AES-SDM) statistical analysis was conducted to determine the mean regions of the brain that were consistently activated. Coordinates from each of the radioligands were first analyzed separately to examine the neural regions involved across each of the neurotransmitters. In order to determine the robustness of each of the studies, a jackknife analysis available through Sdm-Psi was conducted. Analyses from opioidergic and dopaminergic radioligands were then combined to examine the spatial overlap and non-overlap between mu-opioid, general opioid, and D2/D3 receptors using AFNI function 3dcalc (Cox, 1996).
When there were differences in the thresholding in whole-brain and ROI analyses, they were entered separately in order to correct for the threshold differences conducted by the authors. The areas of activation found in the results were shown in high consistency throughout the included studies and the z-scores indicate the likeliness of activation in these areas. Furthermore, a jack-knife analysis was also conducted to account for voxels with only a few studies contributing, this way single studies that could possibly dominate the results would not skew the meta-analysis (Cutler et al., 2018). TFCE (Threshold-free cluster enhancement) analysis was conducted to optimize for cluster-based thresholding. This method improves thresholding sensitivity and prevent smaller clusters from being unaccounted for (Smith and Nichols, 2009). False Discovery Rate (FDR) threshold was set to p < 0.005 in order to assess for random spatial associations between experiments. The standard AES-SDM thresholds (uncorrected voxelwise p-value of p < 0.005, extent threshold clusters for ≥ 10 voxels, and z values of greater than or equal to Z ≥ 1, which are proposed to optimally balance sensitivity and specificity) were used (Lieberman and Cunningham, 2009).
3. Results
3.1. Opioid
To increase power, we combined studies that evaluated selective (μOR) and non-selective (μOR, δOR, and κOR) opioid receptor radioligands. This resulted in a total of 15 articles, with a total n = 283 subjects, yielding 121 foci. When all opioidergic activity was combined, 6 clusters (Table 2) were observed with peak activations in the ventral medial prefrontal cortex, anterior cingulate cortex, right amygdala extending to the temporal pole, right anterior insula, another cluster in the right ventral anterior insula, and a small cluster in the left insula. The largest cluster, with a maximum peak in the ventral medial prefrontal cortex, is composed of 3317 voxel activations spreading across the ventromedial prefrontal cortex and striatum, including bilateral caudate nucleus and thalamus (Figure 2, top).
Table 2.
MNI center of cluster coordinates in each cluster in opioid activations
| x | y | z | Cluster Size Brain Region, Brodmann Area | |
|---|---|---|---|---|
|
| ||||
| 6 | 18 | 4 | 3317 | Ventralmedial PFC, Striatum, Thalamus |
| 0 | −6 | 42 | 1315 | Anterior Cingulate Cortex |
| 22 | 2 | −32 | 962 | Right Amygdala, Right Temporal Pole |
| 40 | 16 | 4 | 394 | Right Insula, Right Inferior Frontal Gyrus |
| 36 | 24 | −10 | 53 | Right Ventral Anterior Insula |
| −34 | 4 | −4 | 18 | Left Insula, Left Putamen |
Threshold Voxel threshold; p < 0.005. Extent threshold; cluster size ≥ 10 voxels.
Figure 2:
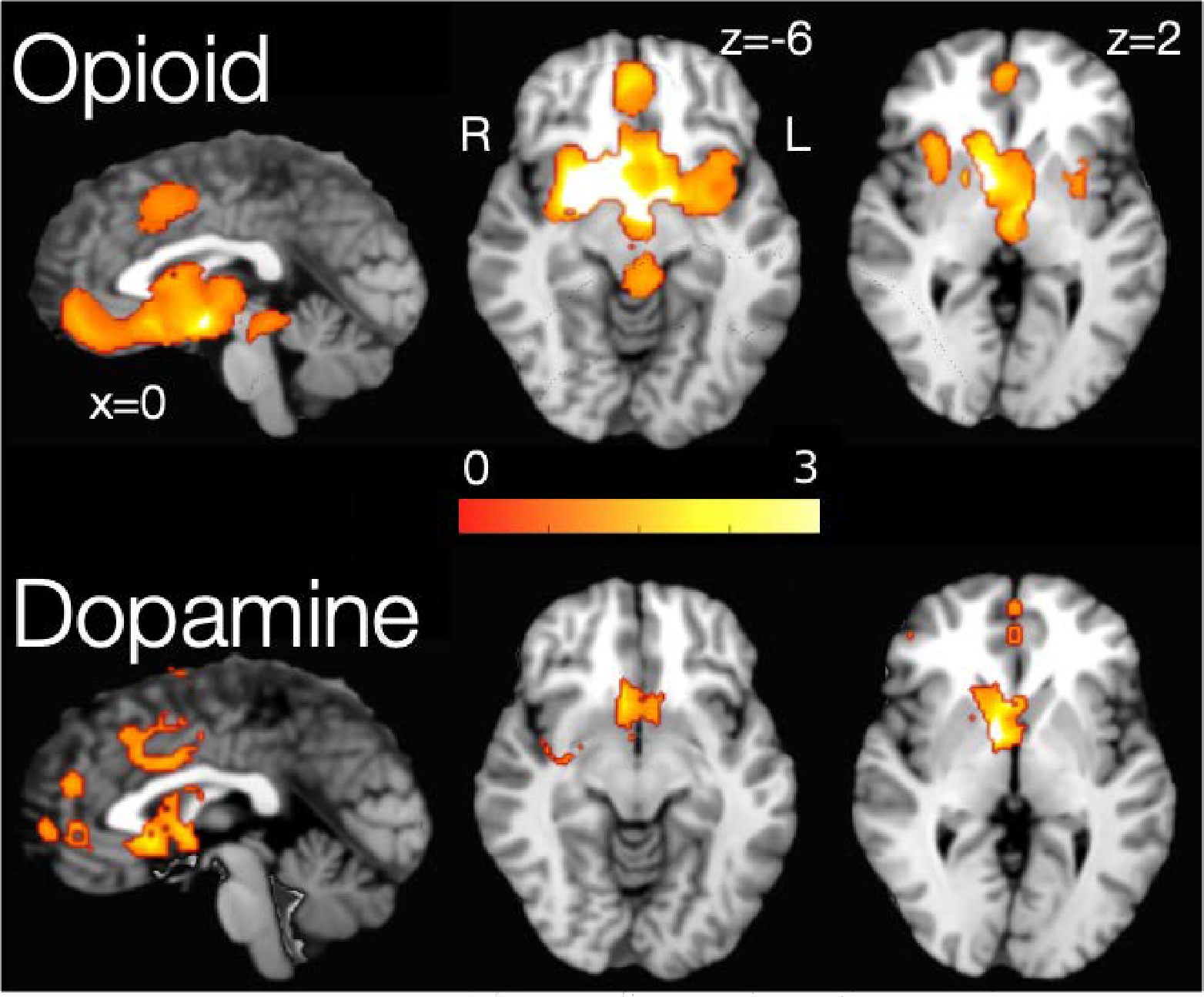
Areas of significant change in the endogenous opioid (top) and dopamine (bottom) transmission during pain-related experiences. Gradient bar represents SDM value.
Jackknife sensitivity analysis revealed that activations in the right caudate, right insula, and the midbrain, were highly robust, as they were replicated in all 15 studies. Areas in the right putamen were highly influenced by Peciña et al., 2015. Opioidergic activations in the inferior frontal gyrus were determined to not be robust, as there was a lack of activation in 7 of the 15 studies (Table 4).
Table 4 Jack.
Knife sensitivity analysis for each significant cluster
| Studies | Right Caudate | Left Caudate | Thalamus | Right Insula | Cingulate Gyrus | Left Insula/STG | Midbrain | Inferior frontal gyrus |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Opioid | ||||||||
|
| ||||||||
| DaSilva, 2014 | y | y | y | y | y | y | y | y |
| Harris, 2009 | y | y | y | y | n | y | y | y |
| Hsu, 2015 | y | y | y | y | y | y | y | y |
| Jones, 1999 | y | y | y | y | y | y | y | y |
| Maarrawi, 2007 | y | y | y | y | y | n | y | y |
| Nascimento, 2014 | y | y | y | y | y | y | y | y |
| Pecina, 2012 | y | y | n | y | y | n | y | n |
| Pecina, 2014 | y | y | y | y | y | n | y | n |
| Prossin, 2015 | y | n | y | y | y | y | y | y |
| Scott, 2007 | y | y | y | y | y | y | y | y |
| Scott, 2008 | y | y | y | y | y | y | y | y |
| Wager, 2007 | y | y | y | y | y | y | y | y |
| Wey, 2014 | y | y | y | y | y | y | y | y |
| Zubieta, 2001 | y | y | y | y | y | y | y | y |
| Zubieta, 2003 | y | n | y | y | n | y | y | y |
|
| ||||||||
| Right Caudate | Left Caudate | Thalamus | Right Insula | Cingulate Gyrus | Left Insula | |||
|
|
||||||||
| Dopamine | ||||||||
|
|
||||||||
| Berman, 2013 | y | y | y | y | y | y | ||
| Jarcho, 2015 | y | y | y | y | n | y | ||
| Martikainen, 2015 | y | y | y | y | y | y | ||
| Pecina, 2014 | y | y | n | y | n | y | ||
| Scott, 2007 | y | y | n | y | n | y | ||
| Scott, 2008 | y | y | y | y | y | y | ||
| Scott, 2006 | y | y | y | y | y | y | ||
| Wood, 2007 | y | y | y | y | n | y | ||
3.2. Dopamine
Eight manuscripts evaluating dopaminergic activity via RAC and FAL reported a total of 49 foci with a total n = 166. Three clusters were observed with peak activations in the right putamen, right caudate nucleus, and left insula (Table 3). The right putamen cluster included 487 voxels and spread across right insula and right globus pallidus (Figure 2, bottom).
Table 3.
MNI center of cluster coordinates for brain regions in D2/D3 activations
| MNI coordinate | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| x | y | z | SDM-Z | P value | Cluster Size | Brain Region |
|
| ||||||
| 34 | 0 | 0 | 4.504 | 0.0010 | 487 | Right Putamen, Right Insula |
| 14 | 10 | 12 | 3.958 | 0.0010 | 443 | Right Caudate Nucleus |
| −36 | 2 | 2 | 3.523 | 0.0040 | 55 | Left Insula, Left Putamen |
Threshold Voxel threshold: p < 0.005. Peak height threshold: peak SDM-Z > 3.128. Extent threshold: cluster size ≥ 55 voxels.
Jackknife sensitivity analysis revealed that activations in the bilateral caudate and bilateral insulae were highly robust, as they were replicated in all eight studies. It should also be noted that activity in the thalamus was highly influenced by Peciña et al., 2014 and Scott et al., 2007. Dopaminergic activations in the cingulate gyrus were determined to not be robust as there was a lack of activation in 4 of the studies (Table 4).
3.3. Overlap
In order to explore co-localization of endogenous opioid and dopaminergic neurotransmission during pain-related aversive experiences, we created a conjunction between opioid and dopamine thresholded meta-analytical maps. The resulting map is displayed in Figure 3. We found biggest overlap within the right striatal regions (Table 5). The striatal cluster covered most of the right caudate nucleus and spread into the right thalamus (Figure 3).
Figure 3:
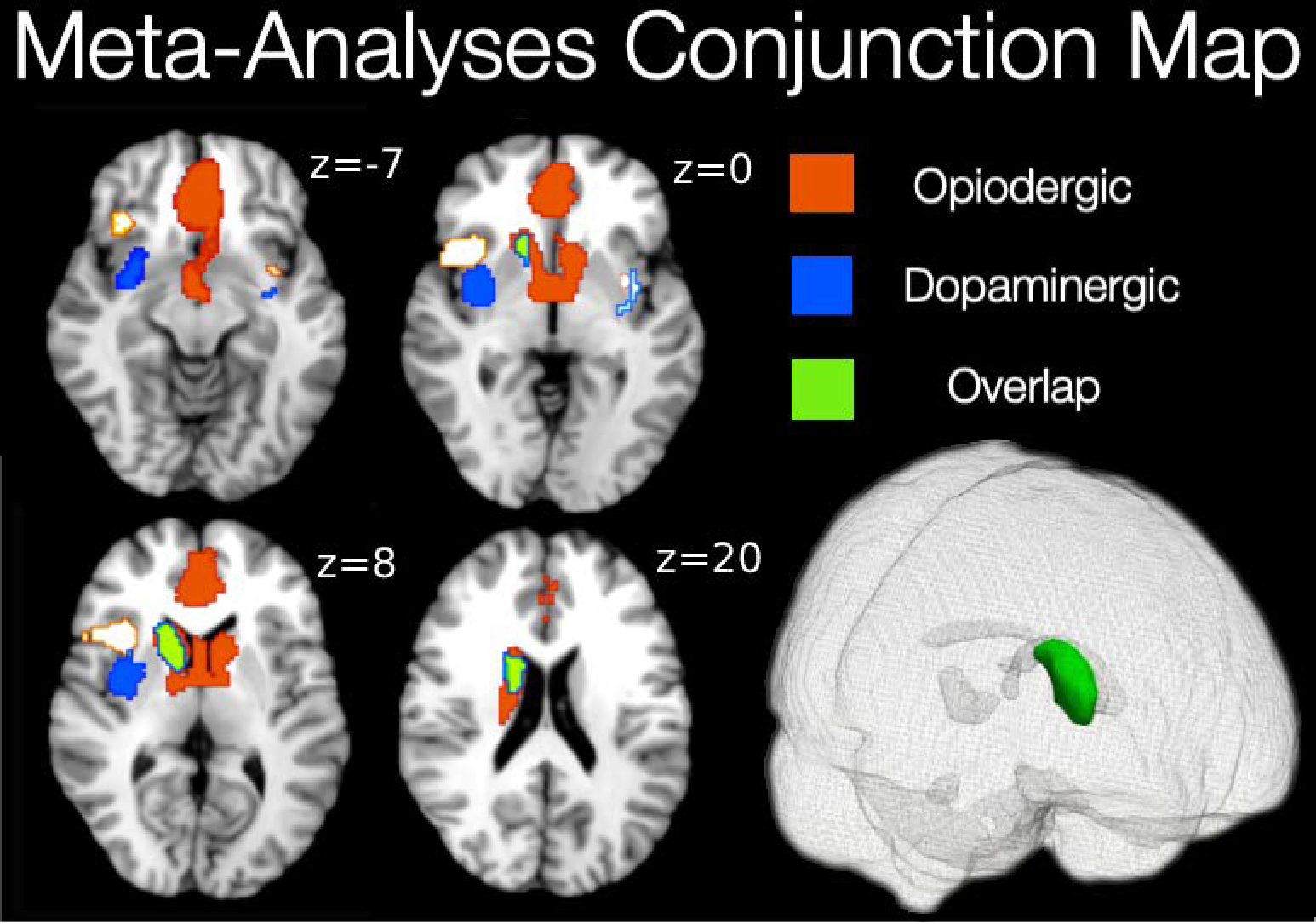
Areas of overlap (green) between endogenous opioid (red) an dopamine (blue) transmission during pain-related experiences.
Table 5.
Peak MNI coordinates for overlap brain regions of opioid and D2/D3 activations
| Peak MNI coordinate | Center of Mass | Cluster Size Brain Region | |||||
|---|---|---|---|---|---|---|---|
| x | y | z | CM x | CM y | CM z | ||
| 14 | 14 | 0 | 13.6 | 9.8 | 11.6 | 420 | Right Dorsal Striatum |
Extent threshold: cluster size ≥ 10 voxels
4. Discussion:
The goal of this investigation was to provide the meta-analytical map of opioidergic and dopaminergic transmissions and their overlap during experiencing aversive conditions including experimental pain, endogenous clinical pain, and emotional pain in humans using the opioidergic and dopaminergic radioligands carfentanil, diprenorphine, raclopride and fallypride. Using the existing literature, we completed two comprehensive meta-analyses of PET radioligand activations using opioidergic and dopaminergic radioligands during painful/aversive experiences.
Consistent with the animal literature, we found pain-related opioidergic activation within striatum, cingulate gyrus extending to supplementary motor area and the midbrain region of the brainstem. The striatal cluster was large and spread across bilateral amygdalae, thalamus, bilateral insulae, subgenual cingulate and frontal pole regions. Our second meta-analysis of dopaminergic activation showed pain -related dopaminergic activation within striatum and cingulate gyrus. The striatal cluster was large and spread across bilateral insulae and the thalamus.
Our primary goal was to identify regions of shared opioid/dopamine neurotransmission during pain-related experiences. The motivation-decision model of pain (Fields, 2018) states that actions are influenced by decisions between whether to approach something pleasant (a reward) or avoid something unpleasant (pain/loss). In this meta-analysis, we potentially shed light on the interaction between pain and pain relief systems in humans and how their associated neurotransmitters, dopamine and opioid respectively, are connected. Given that PET radioligand studies are only able to include one radioligand at a time, it is difficult to study these interactions in humans.
One of the biggest clusters of overlap was found within striatal regions, suggesting that both dopamine and opioid receptor-mediated mechanisms are involved in modulating the perception of aversive experiences related to sensory/emotional pain. The basal ganglia circuits contain one of the highest levels of endogenous opioids and opioid receptors on the brain (McDonald and Lambert, 2005). All opioid receptor subtypes are present in the basal ganglia circuit and their net effect depends on their presynaptic or postsynaptic localization in the different structures of this circuit (Sulzer et al., 2016). Furthermore, both the midbrain VTA and nucleus accumbens receive beta-endorphin–containing projections from the arcuate nucleus and contain enkephalinergic interneurons. Both beta-endorphin and enkephalins, acting via receptors, inhibit GABA release from local inhibitory interneurons, thus facilitating dopamine release in the nucleus accumbens. D2 striatal cells modulate the indirect dopaminergic pathway, which is activated when a stimulus is less rewarding than predicted, suggesting that this is the pathway in which avoidance to an unpleasant stimulus is encoded (Bromberg-Martin et al., 2010). A PET study showed that a single dose of the receptor agonist remifentanil elicited a decrease in the binding of the D2/D3 receptor radiotracer FAL in the ventral striatum, dorsal putamen, and amygdala, reflecting a release of endogenous opioids in both alcohol-dependent patients and controls (Spreckelmeyer et al., 2011). Our results are consistent with co-release of these neurotransmitters in these regions. Along with the existing literature (Kirkpatrick and Bryant, 2015; Reisi et al., 2014; Sulzer et al., 2016), our results suggest that aversive experiences during sensory/emotional pain conditions activates both dopamine and opioid receptors. Our results support the existing literature, in that we found the striatum to have the highest activation in both dopaminergic and opioidergic meta-analyses.
Several limitations should be noted. Our meta-analysis was intended to compare only painful or aversive experimental conditions to understand how opioidergic and dopaminergic systems interact when both hurt and relief conditions are experienced. Due to difficulty and invasive nature of radioligand studies, studies that were included in the current analysis were limited, especially considering limited literature with raclopride and aversive processing. While n=166 is large enough to conduct a meta-analysis, the findings might not be as robust as in our opioid meta-analysis of n=283. In order to increase power and understand aversive-related processing we included studies that examined aversion and hurt related to emotional pain, rather than solely physical pain. In addition to being distressing in nature, social rejection and physical pain have a similar representation in the somatosensory system of the brain (Eisenberger, 2012; Kross et al.). A significant body of research has also established that physical and social pain activate distinct neural representations that are co-localized at the gross anatomical level (Woo et al., 2014; Yarkoni et al., 2011). We are not able to detect patterns of activation in this analyses, only anatomical areas of activation, as the data collected was within-subject peak activation voxels. Given the sex differences in the neurobiology of pain, another limitation of the study involves the inability to conduct a gender analysis due to the small number of studies that provided coordinates for only male or female participants. Finally, pleasure and reward-related processing was not the goal of this study, but should be compared in the future.
5. Conclusion:
To our knowledge, this is the first powerful, data-driven meta-analysis identifying and comparing the neural correlates of opioidergic and dopaminergic transmission in PET radioligand studies of pain. The study design enabled high validity and statistical power by including 449 subjects.
The motivation for the current meta-analysis was to examine the regions in which opioid and dopamine pathways were activated during pain-related and unpleasant conditions, and consequently create a map of the distinct localization of neurotransmitters as well as their overlap. Areas that are known to be consistently activated by pain modulation are the insula, ACC, hypothalamus, PAG, rostral ventral medulla, and spinal cord (Tracey, 2010). After performing a mean analysis of opioid and dopamine binding, we found activity in these previously identified regions, but additionally were able to determine what activity was due to opioid versus dopamine neurotransmission. The significance of the resulting conjunction map (Figure 3) indicates that pain modulation is co-managed by opioid and dopamine receptors in a number of brain regions. Understanding the anatomical arrangement of these types of receptors may help us to further understand the interplay between the neural networks involved in pain modulation and pain relief. By creating maps of opioid and dopamine receptor activation, along with a map of their co-activation during pain modulation, we hope to provide data that can be used to develop targeted pharmaceuticals for patients with pain conditions. For example, animal research on drugs targeting a combination of opioid receptors have found that when targeting δ and μ-opioid receptors, the efficacy of μ-opioid receptors increases and tolerance and dependence are attenuated (Ananthan, 2008). By mapping μ-opioid receptor (CFN) and non-specific opioid receptor (DPN) activation, we hope to provide a deeper understanding of these systems to help assist in the development of future pain treatments.
FUNDING AND DISCLOSURES
This work is supported by a VA Clinical Science Research and Development Merit Grant (I01 CX001762), and, in part, by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number U19AR076737, as well as and Painless Research Foundation.
Footnotes
CONFLICT OF INTEREST STATEMENT
All authors declare no conflicts of interest.
6. References
- Ambrose L, Unterwald E, Van Bockstaele E, 2004. Ultrastructural evidence for co-localization of dopamine D2 and μ-opioid receptors in the rat dorsolateral striatum. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology: An Official Publication of the American Association of Anatomists. 279, 583–591. [DOI] [PubMed] [Google Scholar]
- Ananthan S, 2008. Opioid ligands with mixed μ/δ opioid receptor interactions: an emerging approach to novel analgesics. Drug Addiction: From Basic Research to Therapy. 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Fields HL, Apkarian AV, 2010. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 66, 149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Navratilova E, Porreca F, Borsook D, 2013. Analogous responses in the nucleus accumbens and cingulate cortex to pain onset (aversion) and offset (relief) in rats and humans. J Neurophysiol. 110, 1221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE, 2012. Endogenous opioid systems: current concepts and clinical correlations. Neurology. 79, 807–814. [DOI] [PubMed] [Google Scholar]
- Berman BD, Hallett M, Herscovitch P, Simonyan K, 2013. Striatal dopaminergic dysfunction at rest and during task performance in writer’s cramp. Brain. 136, 3645–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O, 2010. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 68, 815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CA, Matthews J, Fairclough M, McMahon A, Barnett E, Al-Kaysi A, El-Deredy W, Jones AK, 2015. Striatal opioid receptor availability is related to acute and chronic pain perception in arthritis: does opioid adaptation increase resilience to chronic pain? Pain. 156, 2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti A, Searle GE, Long CJ, Hill SP, Reiley RR, Quelch D, Erritzoe D, Tziortzi AC, Reed LJ, Lingford-Hughes AR, 2012. Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biological psychiatry. 72, 371–377. [DOI] [PubMed] [Google Scholar]
- Cowan DT, Wilson-Barnett J, Griffiths P, Allan LG, 2003. A survey of chronic noncancer pain patients prescribed opioid analgesics. Pain Medicine. 4, 340–351. [DOI] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput.Biomed.Res. 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Cutler J, Radua J, Campbell-Meiklejohn D, 2018. Adjusting for Variable Brain Coverage in Voxel-Based fMRI Meta-Analysis. BioRxiv. 457028. [Google Scholar]
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C, 2018. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. Morbidity and Mortality Weekly Report. 67, 1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DD, Kong J, Webb M, Bonab AA, Fischman AJ, Gollub RL, 2008. A combined [11C]diprenorphine PET study and fMRI study of acupuncture analgesia. Behav Brain Res. 193, 63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, 2012. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 13, 421–34. [DOI] [PubMed] [Google Scholar]
- Eriksson O, Antoni G, 2015. [11C] Carfentanil binds preferentially to μ-opioid receptor subtype 1 compared to subtype 2. Molecular imaging. 14, 7290.2015. 00019.. [PubMed] [Google Scholar]
- Fields HL, 2018. How expectations influence pain. Pain. 159 Suppl 1, S3–S10. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS, 2008. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain medicine. 9, 444–459. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Lidow M, Gallager D, 1990. Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. Journal of Neuroscience. 10, 2125–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Nicolini M, Berglind W, Cole K, Keogh C, McGinty J, 2003. Local μ and δ opioid receptors regulate amphetamine-induced behavior and neuropeptide mRNA in the striatum. Neuroscience. 121, 387–398. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Johanson CE, Moody DE, Woods JH, Kilbourn MR, Koeppe RA, Schuster CR, Zubieta JK, 2003. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 28, 2000–9. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Martikainen IK, Mansikka H, Hinkka S, Nagren K, Hietala J, Scheinin H, Pertovaara A, 2002. Dopamine D2 receptor binding in the human brain is associated with the response to painful stimulation and pain modulatory capacity. Pain. 99, 273–9. [DOI] [PubMed] [Google Scholar]
- Harris HN, Peng YB, 2020. Evidence and explanation for the involvement of the nucleus accumbens in pain processing. Neural regeneration research. 15, 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H, Ni L, Walker SJ, Mickey BJ, Korycinski ST, Koeppe RA, Crocker JK, Langenecker SA, Zubieta JK, 2013. Response of the mu-opioid system to social rejection and acceptance. Mol Psychiatry. 18, 1211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalabert M, Bourdy R, Courtin J, Veinante P, Manzoni OJ, Barrot M, Georges F, 2011. Neuronal circuits underlying acute morphine action on dopamine neurons. Proceedings of the national academy of sciences. 108, 16446–16450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Regenbogen C, Ohse MC, Frasnelli J, Freiherr J, Lundström JN, 2016. Brain activations during pain: a neuroimaging meta-analysis of patients with pain and healthy controls. Pain. 157, 1279–1286. [DOI] [PubMed] [Google Scholar]
- Jones AK, Kitchen ND, Watabe H, Cunningham VJ, Jones T, Luthra SK, Thomas DG, 1999. Measurement of changes in opioid receptor binding in vivo during trigeminal neuralgic pain using [11C] diprenorphine and positron emission tomography. Journal of Cerebral Blood Flow & Metabolism. 19, 803–808. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick SL, Bryant CD, 2015. Behavioral architecture of opioid reward and aversion in C57BL/6 substrains. Frontiers in behavioral neuroscience. 8, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klega A, Eberle T, Buchholz H-G, Maus S, Maihöfner C, Schreckenberger M, Birklein F, 2010. Central opioidergic neurotransmission in complex regional pain syndrome. Neurology. 75, 129–136. [DOI] [PubMed] [Google Scholar]
- Kosten TR, George TP, 2002. The neurobiology of opioid dependence: implications for treatment. Science & practice perspectives. 1, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD, Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci U S A. 108, 6270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Nader K, van der Kooy D, 2002. Motivational state determines the functional role of the mesolimbic dopamine system in the mediation of opiate reward processes. Behavioural brain research. 129, 17–29. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA, 2009. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social cognitive and affective neuroscience. 4, 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, Laurent B, Garcia-Larrea L, 2013. Brain opioid receptor density predicts motor cortex stimulation efficacy for chronic pain. PAIN®. 154, 2563–2568. [DOI] [PubMed] [Google Scholar]
- Martikainen IK, Nuechterlein EB, Pecina M, Love TM, Cummiford CM, Green CR, Stohler CS, Zubieta JK, 2015. Chronic Back Pain Is Associated with Alterations in Dopamine Neurotransmission in the Ventral Striatum. J Neurosci. 35, 9957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J, Lambert D, 2005. Opioid receptors. Continuing education in anaesthesia, critical care & pain. 5, 22–25. [Google Scholar]
- Mick I, Myers J, Stokes PR, Erritzoe D, Colasanti A, Bowden-Jones H, Clark L, Gunn RN, Rabiner EA, Searle GE, 2014. Amphetamine induced endogenous opioid release in the human brain detected with [11C] carfentanil PET: replication in an independent cohort. International Journal of Neuropsychopharmacology. 17, 2069–2074. [DOI] [PubMed] [Google Scholar]
- Mueller C, Klega A, Buchholz H-G, Rolke R, Magerl W, Schirrmacher R, Schirrmacher E, Birklein F, Treede R-D, Schreckenberger M, 2010. Basal opioid receptor binding is associated with differences in sensory perception in healthy human subjects: a [18F] diprenorphine PET study. NeuroImage. 49, 731–737. [DOI] [PubMed] [Google Scholar]
- Ong W-Y, Stohler CS, Herr DR, 2019. Role of the prefrontal cortex in pain processing. Molecular neurobiology. 56, 1137–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina M, Love T, Stohler CS, Goldman D, Zubieta J-K, 2015. Effects of the Mu opioid receptor polymorphism (OPRM1 A118G) on pain regulation, placebo effects and associated personality trait measures. Neuropsychopharmacology. 40, 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploner M, Lee MC, Wiech K, Bingel U, Tracey I, 2010. Prestimulus functional connectivity determines pain perception in humans. Proceedings of the National Academy of Sciences. 107, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard H, Llorens-Cortes C, Schwartz J, 1977. Enkephalin receptors on dopaminergic neurones in rat striatum. Nature. 268, 745–747. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D, Phillips ML, El-Hage W, Kronhaus D, Cardoner N, Surguladze S, 2012. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. European psychiatry. 27, 605–611. [DOI] [PubMed] [Google Scholar]
- Reisi Z, Haghparast A, Pahlevani P, Shamsizadeh A, Haghparast A, 2014. Interaction between the dopaminergic and opioidergic systems in dorsal hippocampus in modulation of formalin-induced orofacial pain in rats. Pharmacology Biochemistry and Behavior. 124, 220–225. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Koeppe RA, Zubieta JK, 2007. Time-course of change in [11C]carfentanil and [11C]raclopride binding potential after a nonpharmacological challenge. Synapse. 61, 707–14. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK, 2008. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 65, 220–31. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE, 2009. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 44, 83–98. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Paulzen M, Raptis M, Baltus T, Schaffrath S, Van Waesberghe J, Zalewski MM, Rösch F, Vernaleken I, Schäfer WM, 2011. Opiate-induced dopamine release is modulated by severity of alcohol dependence: an [18F] fallypride positron emission tomography study. Biological psychiatry. 70, 770–776. [DOI] [PubMed] [Google Scholar]
- Sprenger T, Valet M, Boecker H, Henriksen G, Spilker ME, Willoch F, Wagner KJ, Wester HJ, Tolle TR, 2006. Opioidergic activation in the medial pain system after heat pain. Pain. 122, 63–7. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Cragg SJ, Rice ME, 2016. Striatal dopamine neurotransmission: regulation of release and uptake. Basal ganglia. 6, 123–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, 2010. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med. 16, 1277–83. [DOI] [PubMed] [Google Scholar]
- Tuominen L, Tuulari J, Karlsson H, Hirvonen J, Helin S, Salminen P, Parkkola R, Hietala J, Nuutila P, Nummenmaa L, 2015. Aberrant mesolimbic dopamine–opiate interaction in obesity. NeuroImage. 122, 80–86. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler J, Wang G, Baler R, Telang F, 2009. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 56, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wey HY, Catana C, Hooker JM, Dougherty DD, Knudsen GM, Wang DJ, Chonde DB, Rosen BR, Gollub RL, Kong J, 2014. Simultaneous fMRI-PET of the opioidergic pain system in human brain. Neuroimage. 102 Pt 2, 275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoch F, Schindler F, Wester HJ, Empl M, Straube A, Schwaiger M, Conrad B, Tolle TR, 2004. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: a [11C]diprenorphine PET study. Pain. 108, 213–20. [DOI] [PubMed] [Google Scholar]
- Wiskerke J, Schetters D, van Es IE, van Mourik Y, den Hollander BR, Schoffelmeer AN, Pattij T, 2011. μ-Opioid receptors in the nucleus accumbens shell region mediate the effects of amphetamine on inhibitory control but not impulsive choice. Journal of Neuroscience. 31, 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Koban L, Kross E, Lindquist MA, Banich MT, Ruzic L, Andrews-Hanna JR, Wager TD, 2014. Separate neural representations for physical pain and social rejection. Nat Commun. 5, 5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA, 2007. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 25, 3576–82. [DOI] [PubMed] [Google Scholar]
- Wood PB, 2008. Role of central dopamine in pain and analgesia. Expert Rev Neurother. 8, 781–97. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD, 2011. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 8, 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS, 2001. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 293, 311–5. [DOI] [PubMed] [Google Scholar]


