Abstract
We suggest that to understand complex behaviors associated with fear and anxiety, we need to understand brain processes at the collective, network level. But what should be the type and spatial scale of the targeted circuits/networks? Not only are multi-region interactions essential—including complex reciprocal interactions, loops, and other types of arrangement—but it is profitable to characterize circuits spanning the entire neuroaxis. In particular, it is productive to conceptualize the circuits contributing to fear/anxiety as embedded into large-scale connectional systems. We discuss circuits involving the basolateral amygdala that contribute to aversive conditioning and fear extinction. In addition, we highlight the importance of the extended amygdala (central nucleus of the amygdala and bed nucleus of the stria terminalis) cortical-subcortical loop, which allows large swaths of cortex and subcortex to influence fear and anxiety. In this manner, fear/anxiety can be understood not only based on traditional “descending” mechanisms involving the hypothalamus and brainstem, but in terms of a considerably broader reentrant organization.
Keywords: fear, anxiety, extended amygdala, bed nucleus of the stria terminalis, networks
1. Introduction
Neuroscientists seek to understand the neural basis of mental functions. What will it take to understand the brain basis of fear and anxiety? Clearly, understanding one or two brain regions won’t be enough—we need to study these constructs at the circuit or network level (we’ll use the terms “circuit” and “network” more or less interchangeably, and in a functional sense, not anatomically). But even here, it’s not clear what spatial scales are most profitable. And if the answer is a circuit or network, what kind of circuit should be considered? And what does it mean to study fear and anxiety at the network level?
Before proceeding, it is worth saying a few words about the terms “fear” and “anxiety”. In fear, typically, the danger is imminent, mostly unambiguous, such that the animal is mobilized for immediate action, including fight or flight. In anxiety, typically, threats are more uncertain and diffuse. It’s often emphasized that anxiety involves a lasting state of apprehension of potential future threats, accompanied by negative affect, autonomic symptoms, worry, increased vigilance, and passive avoidance.
However, the above conceptualization, common as it is, is problematic because it encourages a fairly binary division of labor. As developed elsewhere (Pessoa et al., 2022), whereas mental terms can be at times useful in orienting researchers along research avenues, they are generally inadequate in conveying the interdependence of mental processes. The discussion of neural circuits below should help illustrate how neural circuits do not respect boundaries typically adopted by investigators. Accordingly, “fear” and “anxiety” are used as placeholders but should not be understood as dichotomous constructs that map to separate neural circuits.
In the present piece, most of the literature on the neural basis of fear and anxiety is described based on the rodent literature, which provides a more comprehensive picture of the circuits involved. Human studies paint a somewhat similar picture, but space limitations preclude a detailed interspecies comparison. Nevertheless, the ensuing discussion applies across species.
2. Circuits involved in fear-related processing
For brevity, we will illustrate fear-related processing with classical conditioning circuits (LeDoux, 2000; Maren, 2001; Tovote et al., 2015). As stated above, we do not mean to imply a strict separation of “fear” and “anxiety”, which we believe is counterproductive. This should not be surprising, after all brain circuits have been shaped by evolution to solve behavioral problems that promote survival. Mental terms used by human researchers are, consequently, poor descriptors that have more to do with research traditions than anything else.
A critical node of classical conditioning circuits is the amygdala, a highly heterogenous complex with a dozen or more anatomical subdivisions. Here, we will focus on the basolateral amygdala and the central nucleus of the amygdala. The central nucleus targets multiple areas along the basal forebrain and brainstem that mobilize the neuroendocrine and autonomic systems. How does the central nucleus itself get engaged? During conditioning, the lateral component of the basolateral amygdala is essential for learning the association between the conditioned stimulus (CS+; which initially is benign) and the unconditioned stimulus (US; inherently aversive). Once learning solidifies, the basolateral amygdala engages the central nucleus when a CS+ is encountered (Figure 1A). The circuit described thus far, although building upon local amygdala micro-circuits, can be considered essentially unidirectional: sensory signals linked to the CS+ impinge on the basolateral amygdala, which engages the central amygdala, with outflow to the hypothalamus and multiple sites along the brainstem. Indeed, this descending organization of fear (and anxiety) circuits is a hallmark of existing proposals.
Figure 1.
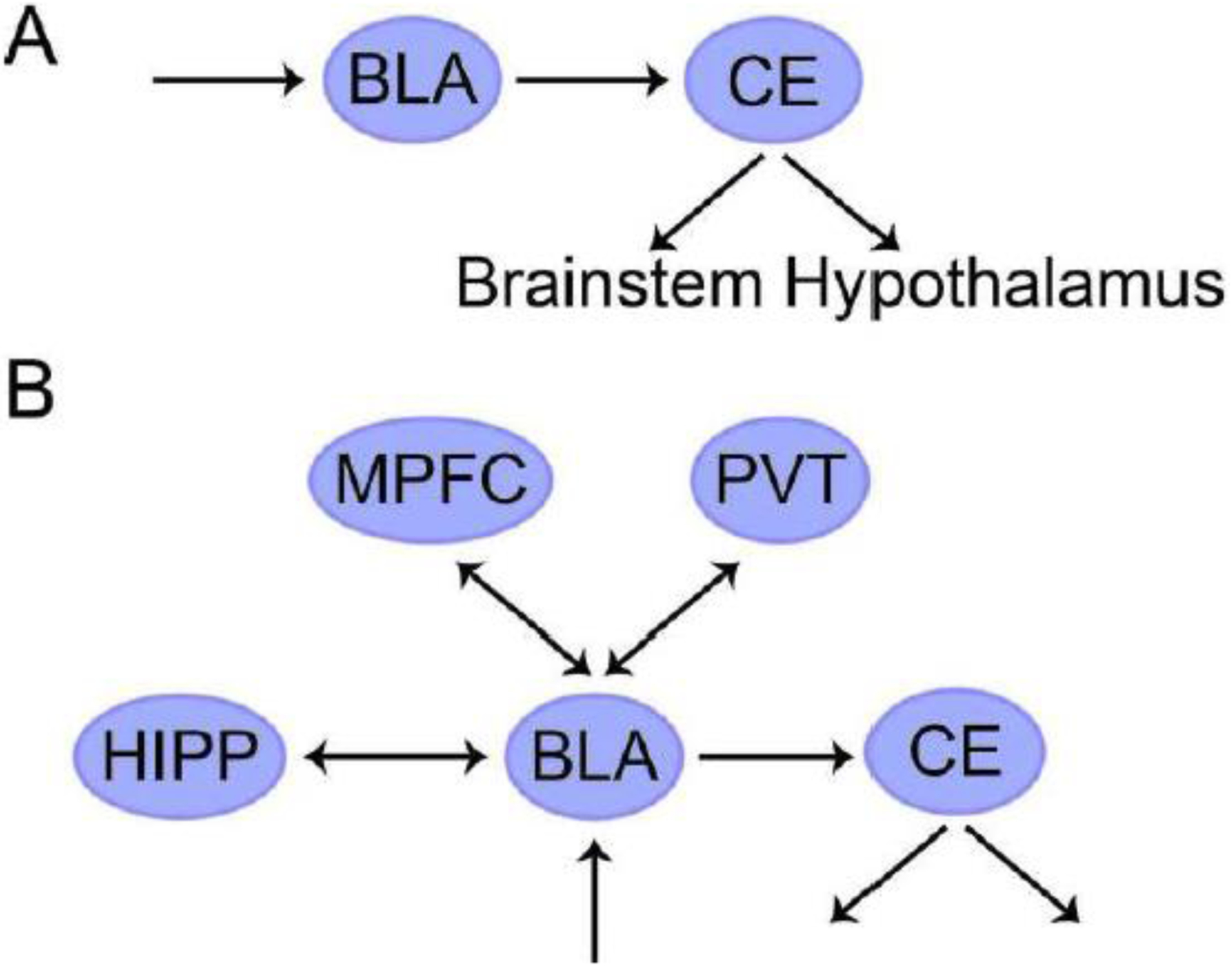
Fear circuits. (A) Traditional circuit focusing on the descending engagement of autonomic and neuroendocrine responses. (B) Expanded circuit with bidirectional connections.
Abbreviations: BLA, basolateral amygdala; CE, central nucleus of the amygdala; HIPP, hippocampus; MPFC, medial prefrontal cortex; PVT, paraventricular nucleus of the thalamus.
Although the basic circuit described elucidates key components of aversive conditioning, the circuit needs to be extended (Figure 1B). For example, conditioning based on more complex auditory stimuli is abolished when auditory cortex circuits are blocked (Letzkus et al., 2011). Even in response to simple auditory conditioned stimuli, fear expression depends on the prelimbic cortex in medial prefrontal cortex (PFC; dorsomedial PFC in humans) (Corcoran and Quirk, 2007). Furthermore, the prelimbic cortex and the basal amygdala are reciprocally connected and exhibit entrainment of theta rhythms after conditioning (Likhtik et al., 2014). Bidirectional interactions also exist between the basolateral amygdala and the hippocampus, a region that processes context-related information. This is important because fear responses can be context dependent (see below on extinction). Finally, the paraventricular nucleus of the thalamus (PVT) is required for the expression of remote but not recent fear memories (Do-Monte et al., 2015). Because the last three regions discussed—prelimbic cortex, hippocampus, and PVT—are bidirectionally connected with the basolateral amygdala, the coordinated activity between all of them likely plays a notable role in fear-related processing.
The discussion thus far illustrates the need to consider a broader set of brain regions in studying fear-related processing. (For brevity, we have omitted additional regions, including the periaqueductal gray, which conveys signals about the US to the central amygdala; Johansen et al., 2010). But it is necessary to go beyond the “minimal circuit” in Figure 1B because fear needs to be understood both in terms of processing that promotes fear and processing that opposes it. Regarding the latter, for example, fear extinction processes eventually transform a fear-inducing CS+ stimulus into one that is (mostly) neutral (Dunsmoor et al., 2015; Bouton et al., 2021). Thus, in all but the simplest laboratory settings, fear-promoting and -opposing processes are at play and need to be considered to explain behavior.
Let us briefly consider extinction. Both behaviorally and neurobiologically, fear extinction is now understood to be rather complex (Dunsmoor et al., 2015; Bouton et al., 2021). Early models of extinction emphasized the role of the medial PFC (infralimbic cortex in rodents and ventromedial PFC in humans) in modulating the basolateral amygdala to dampen fear in the face of a now-extinguished stimulus—a previous CS+ that, through extinction learning, now is associated with safety (Figure 2A). As the safety of a previous CS+ critically depends on environmental context, the hippocampus was viewed as important, too. Such early models have been substantially updated, and the emerging picture is considerably more elaborate (Figure 2B). A key development has been the realization that the medial PFC works in a coordinated fashion with the basolateral amygdala (the two are bidirectionally connected), such that the initial idea that the former (associated with “cognition” in cortex) simply dampens the latter (associated with “emotion” in subcortex) is problematic (Figure 2A). Some studies even suggest that the basolateral amygdala is “upstream” of the medial PFC, as a population of “extinction neurons” in the basolateral amygdala increase their activity during extinction learning (Herry et al., 2008), and contribute to medial PFC activity.
Figure 2.
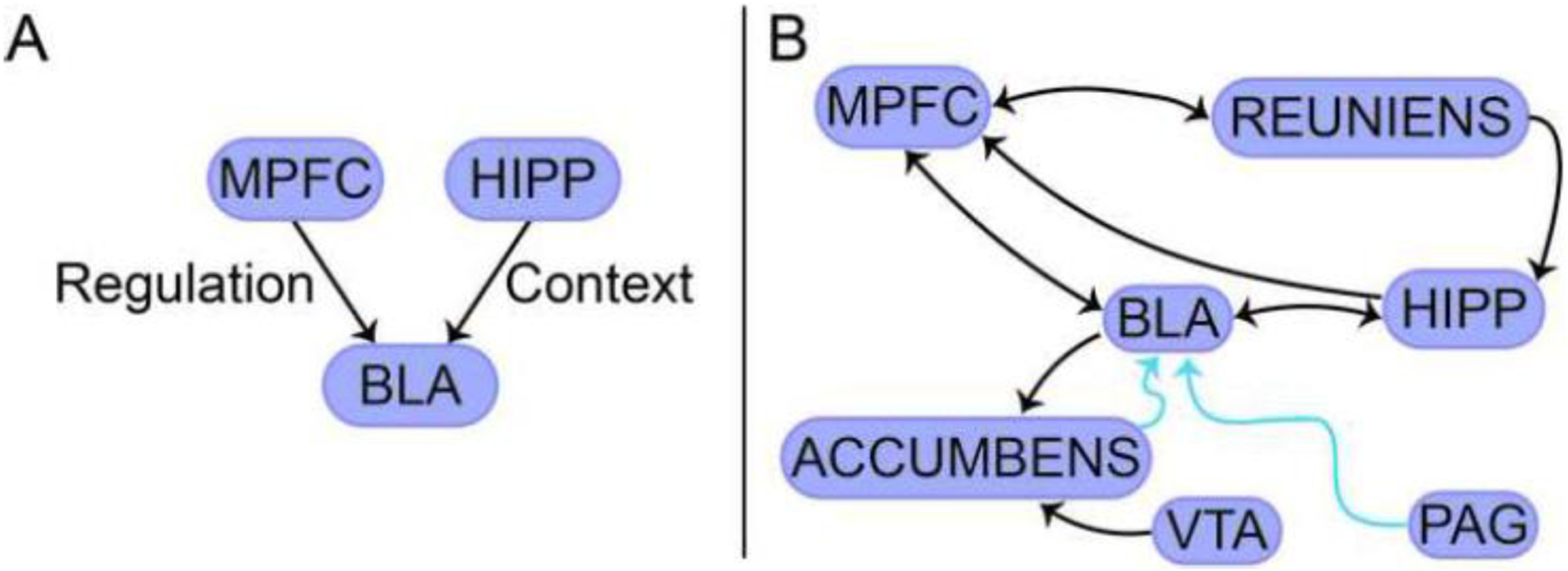
Fear extinction circuits. (A) Basic circuit focusing on regulation of the amygdala by the medial prefrontal cortex. (B) Expanded circuit with bidirectional connections. Reuniens is a nucleus of the thalamus. Arrows in blue represent indirect connections.
Abbreviations: PAG, periaqueductal gray; VTA, ventral tegmental area. See also Figure 1.
To reiterate, the elucidation of the neural basis of fear requires working out the promotion of fear as well as the dampening of fear. The circuits are often studied separately, but should be considered jointly for a comprehensive view of fear processing.
3. Circuits involved in anxiety-related processing
Circuits involved in anxiety-related processing overlap with those in fear but differences must be highlighted. One of the most noteworthy concerns the bed nucleus of the stria terminalis (BST; also abbreviated as BNST). In an influential model, the BST was proposed to be engaged by diffuse threat, whereas the central amygdala was proposed to be engaged by immediate threat (Davis and Whalen, 2001). This purported dissociation has been challenged by some researchers (Gungor and Pare, 2016; Shackman and Fox, 2016; Fox and Shackman, 2019), but the precise contributions of the BST and the central amygdala to sustained versus phasic processes, respectively, remains unresolved (for examples in humans, see Hur et al., 2020; Murty et al., 2022).
Another brain region central to anxiety-related processes is the insula. Indeed, in the human literature, models of anxiety often concur in emphasizing the key role of this region, especially the anterior sector (Paulus and Stein, 2007; Grupe and Nitschke, 2013). Intriguingly, frameworks centered on rodents emphasize subcortical contributions (with the clear exception of the medial PFC), such that the insula in many cases is entirely missing, although recent studies have started to investigate the contributions of this region in rodents, too (Klein et al., 2021). At present, the roles of the insula in anxiety remain somewhat unclear, but have been proposed to include heightened responses during the anticipation of aversive events and the evaluation of risk. In addition, the insula is believed to generate anticipatory responses in the face of hypothetical future events so as to answer the following question: “how is it going to feel?” (Grupe and Nitschke, 2013).
From a broader perspective, uncovering the neural basis of anxiety poses multiple challenges. To see why, consider multiple ways in which threat-related processing is believed to contribute to maladaptive behaviors in humans with anxiety disorders (Grupe and Nitschke, 2013): inflated estimates of threat magnitude and probability, hypervigilance, deficient safety learning, behavioral and cognitive avoidance, and heightened reactivity to threat uncertainty. The broad range of these processes demonstrates the multifaceted nature of “anxiety”—ultimately, a broad umbrella term. Consequently, we can say that there is no “anxiety network” (singular), as much as a variety of circuits that contribute to anxiety-related behavioral manifestations.
4. Embedding circuits into large-scale systems
The multifaceted aspects of fear and anxiety motivate understanding them in an even broader anatomical-functional perspective. Accordingly, it is profitable to situate them in terms of the connectional logic of the neuroarchitecture that interlinks the multiple sectors of the neuroaxis (Pessoa et al., 2019, discusses how this organization is present across most vertebrates). A key element of this organization is the presence of cortical-subcortical-cortical loops, as exemplified by basal ganglia loops.
Basal ganglia loops are a defining feature of the architecture of the vertebrate brain (except in fishes). In mammals, the entire cortical sheet projects to the striatum and loops back to the cortex via the thalamus (Figure 3A). An important feature of mammalian basal ganglia loops is that they involve both dorsal (caudate-putamen) and ventral (nucleus accumbens) striatal components. Some cortical areas project to the dorsal striatum (for example, motor and somatosensory areas), while others project to the ventral striatum (in primates, for example, orbitofrontal, prefrontal, and anterior cingulate cortices).
Figure 3.
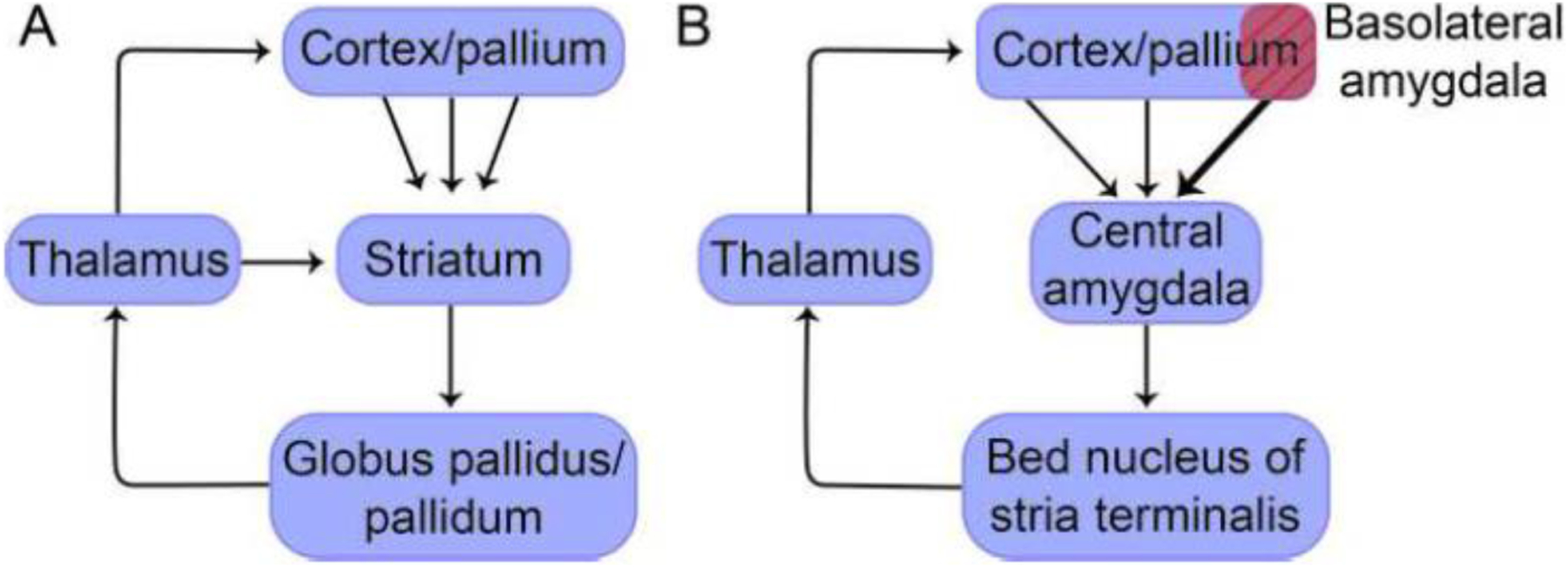
Cortical-subcortical loops are an important principle of macro-scale anatomical organization. (A) Standard basal ganglia loops. All sectors of the cortex project to the striatum, looping back via the thalamus. (B) The extended amygdala loop has a comparable overall organization. Note that the strongest projection from the cortex/pallium is from the basolateral amygdala which is substantially more pronounced than that of other sectors. Line thickness of the connections between the cortex/pallium to the central amygdala conveys pathway weight.
Notably, a similar connectional logic is observed involving other parts of the basal forebrain, of which we emphasize cortical-subcortical loops engaging the “extended amygdala”. The extended amygdala concept developed by Alheid and Heimer (1988) considers the central amygdala and the BST to be a basal-ganglia-like anatomical-functional unit. To appreciate this organization, it is important to consider the two major components of the forebrain: the pallium and the subpallium. During embryological development, the pallium gives rise not only to the entire cortex but also to the basolateral amygdala, whereas the subpallium gives rise to the subcortex, including the central amygdala and the BST. Thus, we see that the latter two regions belong to a qualitatively different sector of the brain compared to the basolateral amygdala (and cortex). In addition, based on cell types and molecular profiles, the central amygdala is a striatum-like region, whereas the BST is a pallidum-like region (in mammals, the pallidum corresponds to the globus pallidus).
With the above facts in mind, now it should be possible to follow the extended amygdala loop (Figure 3B). The basolateral (pallial) amygdala interfaces with the extended amygdala much like the cortex interfaces with standard basal ganglia loops (functionally, this also matches the integrative properties of the pallial amygdala, which receives massive inputs from across the cortex; see below). The central amygdala (striatum-like region) projects to the BST (pallidum-like region). The BST subsequently projects to the thalamus, which in turn projects to cortical targets. The pathways from the BST to the thalamus target the PVT and other midline nuclei. In all, the overall arrangement establishes a pathway through the central extended amygdala and back to the cortex/pallium. For a detailed exposition of the extended amygdala system (Figure 3B), see Heimer et al. (2007).
Standard basal ganglia loops (via the striatum) play a major role in the flow of cortical signaling. Classically linked to movement control and disorders, the basal ganglia are now known to be involved in cognition, motivation, and emotion, and viewed as essential for higher level behavioral control, including learning and regulation of stimulus-driven behaviors, as well as action selection supporting goal-directed behaviors (Yin and Knowlton, 2006; DeLong and Wichmann, 2009; Nelson and Kreitzer, 2014). We propose that the extended amygdala loop should be conceptualized as contributing to a broad and diverse set of cognitive-emotional-motivational processes, too.
What is the importance of the extended amygdala loop in the case of fear and anxiety? Traditionally, conceptualizations of fear and anxiety circuits center around two key properties. First, they are highly centralized. For example, fear circuits are centered on the basolateral and central amygdala, and anxiety circuits are centered on the BST. Second, they are built around the idea of descending control (Figure 4A). For example, both the central amygdala and the BST assemble autonomic and endocrine responses by engaging the hypothalamus and brainstem (historically, the hypothalamus itself has been conceptualized as a “master controller” of the autonomic system). The reentrant organization of the extended amygdala loop suggests a complementary view that places fear- and anxiety-related processing as embedded within broader cognitive-emotional-motivational circuits (Figure 4B).
Figure 4.
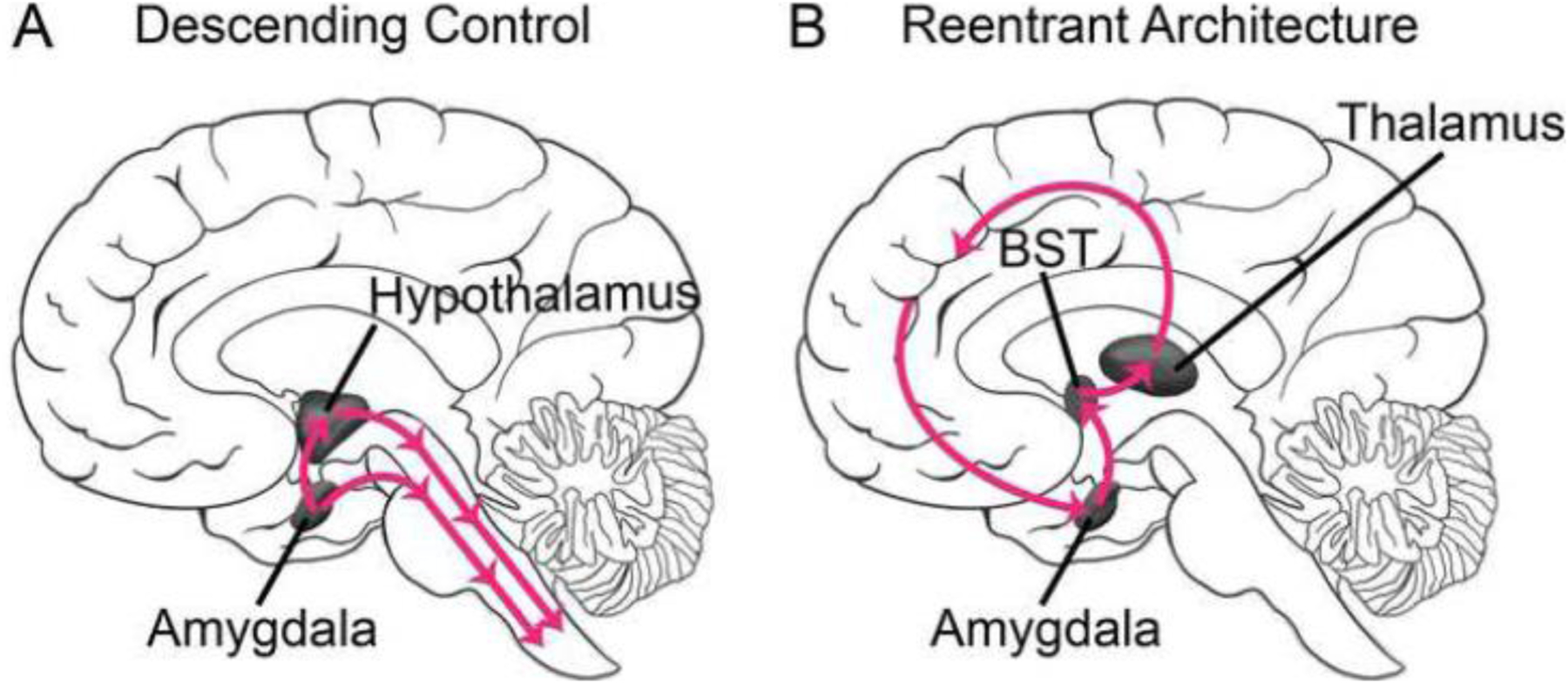
Contrasting organizations. (A) Traditional view in terms of centralized processing and descending control. (B) Complementary proposal in which the reentrant organization of the extended amygdala loop plays a key role.
To further motivate this idea, consider the relationship between cortical-subcortical reentrant systems. Classical basal ganglia loops (via the striatum) are viewed as rather independent and parallel. However, accruing evidence points to considerable crosstalk between these systems, with substantial signal intermixing (Joel and Weiner, 1994; Haber, 2010; Hintiryan et al., 2016; Groenewegen et al., 2016; Aoki et al., 2019). We thus propose that they be viewed as distributed, intercommunicating systems that provide integral contributions to cortical function. How about the extended amygdala loop? The cortical-like component of the loop is the basolateral amygdala, which is bidirectionally connected with essentially the entirety of the cortex (albeit with different connectivity strengths). This organization shows that the extended amygdala loop has unique potential to contribute to overall brain function.
As stated, classical basal ganglia loops are not independent and have multiple opportunities to communicate with one another. Remarkably, these loops are interlinked with the extended amygdala loop, too. We suggest this is a significant arrangement because it allows the circulation of disparate signals (action-related, emotional, motivational, and so on) across multiple loops, considerably broadening the range of signal distribution and integration. In particular, the PVT is well-positioned to interlink systems (Kirouac, 2015) (Figure 5). This thalamic nucleus projects to both the central extended amygdala and the nucleus accumbens, and is reciprocally connected with pallial areas, such as the insular cortex, the prefrontal cortex (including orbitofrontal cortex), the hippocampal formation, and the basolateral complex of the amygdala (these pallial sectors are reciprocally interconnected and project to the central extended amygdala and nucleus accumbens) (reviewed by Kirouac, 2015, 2021). Notably, individual PVT neurons have axons that bifurcate to innervate multiple targets (Unzai et al., 2015; Dong et al., 2017). Most neurons in the PVT project to the nucleus accumbens, but a significant proportion send collaterals to the BST and the central amygdala. In addition, neurons that project to the nucleus accumbens, BST, and central amygdala are intermixed throughout the PVT and do not appear to form clusters of unique subpopulations of projection-specific neurons.
Figure 5.
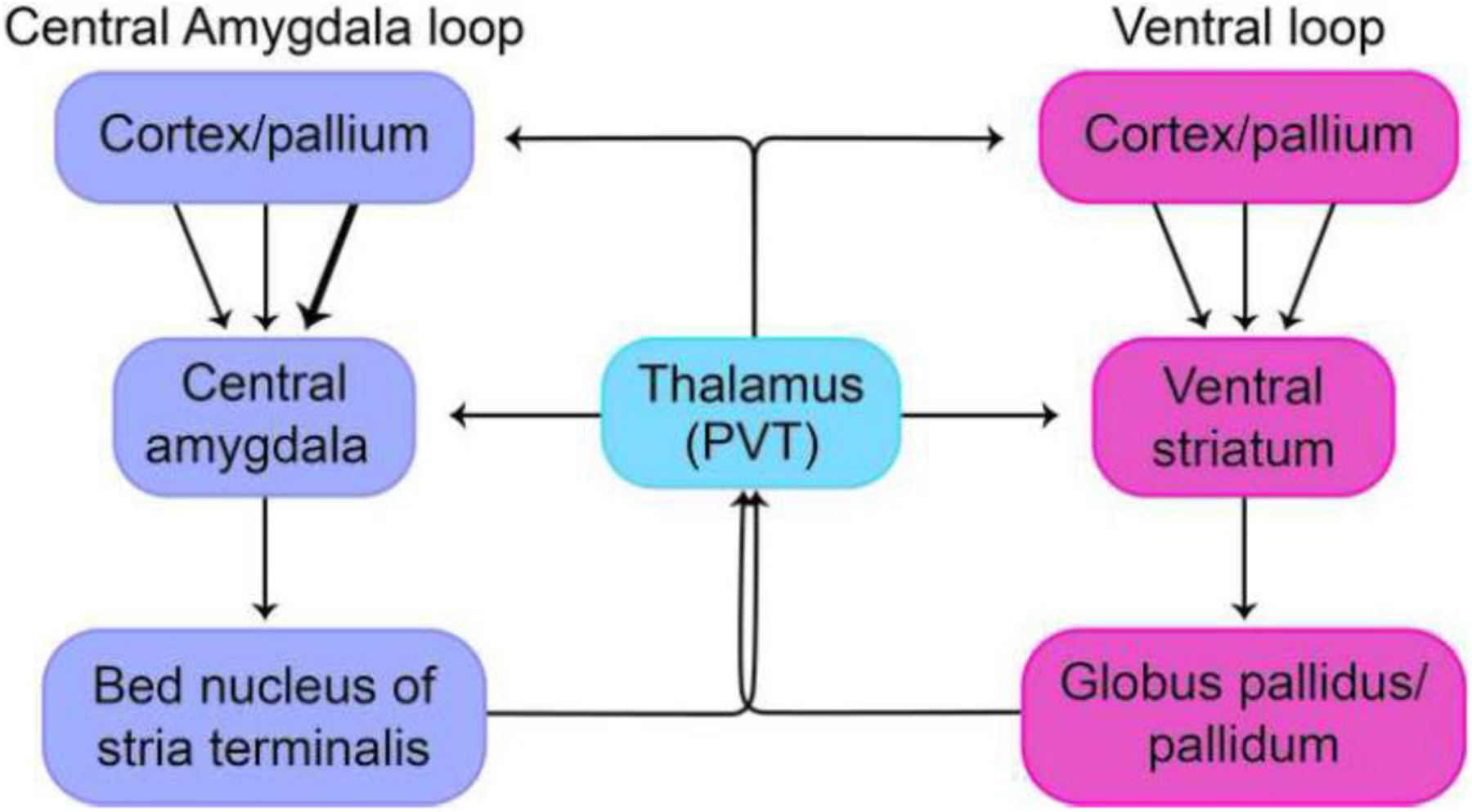
Large-scale connectional system intercommunication. The paraventricular nucleus of the thalamus (PVT) serves as a hub region that interlinks the central amygdala loop with the standard basal ganglia ventral loop, both at the level of the thalamus and via the return to cortex/pallium.
To conclude this section, a brief aside on network science. Hub regions are highly connected ones that have the potential to play chief roles in signal distribution and integration, with connector hubs having particular importance in interlinking disparate parts of the system (Figure 6A). Here, we propose to extend this notion to hub circuits, those that have strong potential in influencing processing in disparate brain sectors (Figure 6B). We propose that the extended amygdala loop is one such hub circuit. Given the expedient access that the central amygdala and the BST have to neuroendocrine and autonomic functions (via the hypothalamus and brainstem), the loop places extended amygdala function in very close association with the cortex. In this context, attempting to separate emotion, motivation, and cognition becomes a largely problematic exercise.
Figure 6.
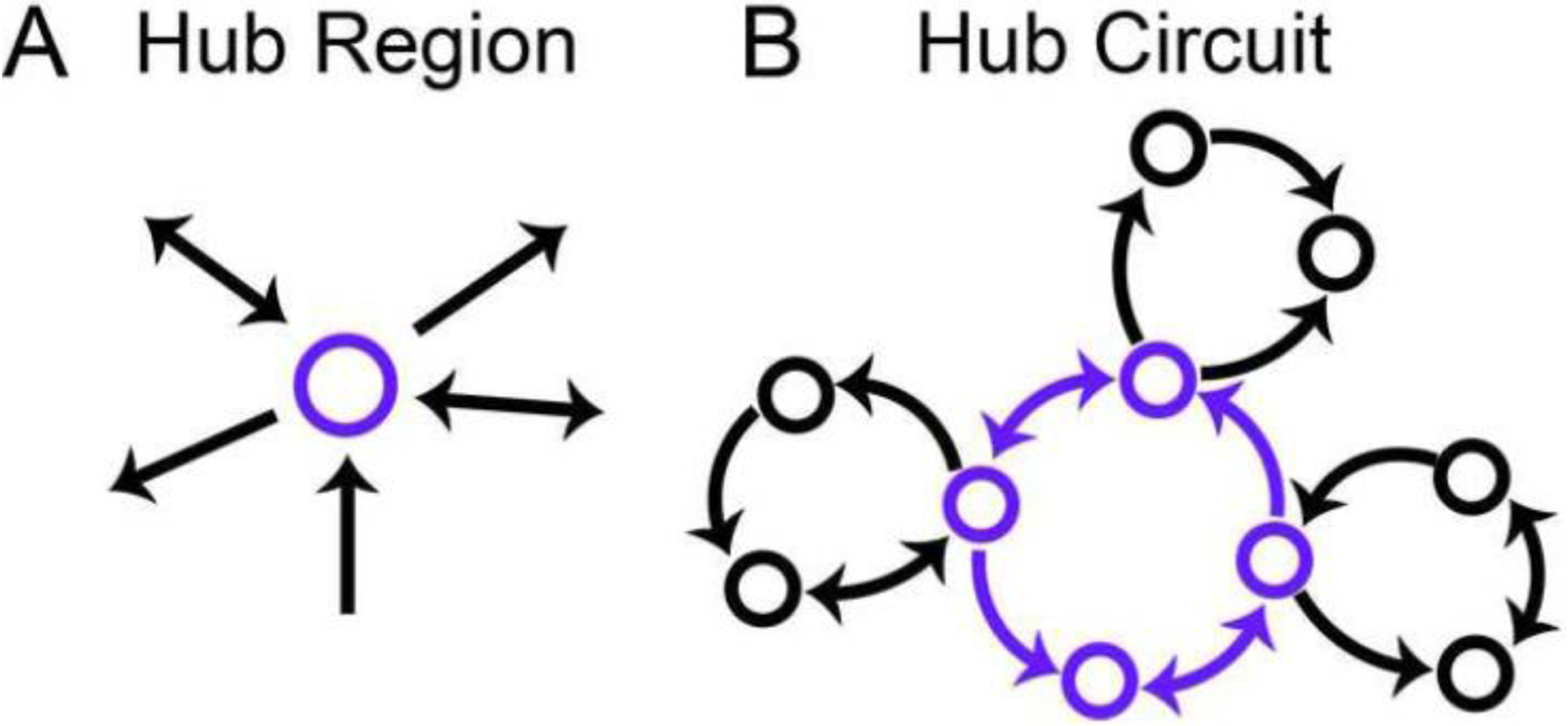
Hubs in the brain. (A) Hub regions are highly connected. (B) Hub circuits are proposed to be functional units that can be engaged by or engage multiple circuits.
5. Where next?
The upshot of the ideas developed here is that to understand complex behaviors we associate with fear and anxiety, we need to understand brain processes at the collective, network level. Multi-region interactions are essential, including complex reciprocal interactions, loops, and other types of arrangement.
So, what are network properties? When the functional unit of interest is apparent only when one considers the system, but not its component parts, we can say that we have a network-level property. The case made in the present piece is that in studying fear and anxiety it will not only be profitable but necessary to consider multiregion functions. For further discussion, please see Pessoa (2014; 2022).
What are additional implications of the ideas described in the present article? Neuroscience is experiencing a methodological renaissance. Advances in chemistry and genetics now allow precision in targeting regions and circuits in ways that would have been impossible a decade ago. Multiple developments permit recording over a larger number of regions simultaneously. We believe such methods will be essential in advancing the study of fear and anxiety. We have argued elsewhere that it will be important to also develop techniques that enable multiregion perturbations, including activating and/or silencing multiple regions simultaneously (Pessoa, 2022). We suggest that perturbation experiments could be used to test the contributions of the extended amygdala cortical-subcortical loop. In particular, inactivating the “return loop” via the thalamus to the cortex/pallium is anticipated to strongly compromise the function of the circuit given the broad contributions of the return connections to cortical/pallial signals.
Another recommendation is that the field needs to adopt more dynamic, richly contextual, and naturalistic experimental designs. In such settings, we believe that the functions of large-scale systems discussed here will prove informative. This is because the brain’s considerable anatomical-functional interactional complexity parallels the enormous richness of animal behavior (Pessoa et al., 2022). Typical laboratory settings have severely limited what can be studied. For example, a type of behavior that “fits inside a box” is classical conditioning, which has been repeatedly examined since the early twentieth century. Limited in-a-box behaviors have also been studied to inform anxiety-related processes. These experimental manipulations offer a window into a few dimensions of fear and anxiety while allowing careful control over study variables. But the fixation with simple tasks has led to a form of tunnel vision. As Dennis Paré and Gregory Quirk, prominent researchers in this area, state in the context of classical conditioning:
When a rat is presented with only one threatening stimulus in a testing box that allows for a single reflexive behavioral response, one is bound to find exactly what the experimental situation allows: neuronal responses that appear tightly linked to the CS and seem to obligatorily elicit the conditioned behavior. (Paré and Quirk 2017, 6)
Placed inside a small, enclosed chamber the animal is limited to a sole response: Upon detecting the CS+, it ceases all overt behavior and freezes in place (see also Holley and Fox, 2022). It can’t consider other options, such as dashing to a corner to escape; it cannot try to attack the source of threat either, as there is no other animal around—the shock comes out of nowhere! Now, when researchers study the rat’s brain under such conditions, a close relationship between brain and behavior is established. But as Paré and Quirk warn, the tight link might be apparent insofar as it would not hold under more general conditions.
Thus, while critical, the use of novel neurotechniques mentioned above is insufficient. If we continue using the paradigms that have been the mainstay of the field, we will be cornering ourselves into a scientific cul-de-sac. It is time to think outside the box. Fortunately, more naturalistic paradigms are now possible given recent technical advances. And if we follow novel research paths, it will become apparent that the question of how many brain regions are needed to understand the neural basis of fear and anxiety is actually ill-posed. We need to study a large set of intersecting circuit interactions to make progress.
Highlights.
Complex behaviors associated with fear/anxiety require network-level explanations
Basolateral amygdala circuits contribute to aversive conditioning and fear extinction
We highlight the importance of the extended amygdala cortical-subcortical loop
Circuit allows large swaths of cortex and subcortex to influence fear and anxiety
Acknowledgements
The author acknowledges research support from National Institute of Mental Health (MH071589 and MH112517). The author is also grateful for the constructive feedback provided by reviewers, which considerably improved the text.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alheid GF, & Heimer L (1988). New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience, 27(1), 1–39. [DOI] [PubMed] [Google Scholar]
- Aoki S, Smith JB, Li H, Yan X, Igarashi M, Coulon P, Wickens JR, Ruigrok TJH, Jin X. 2019. An open cortico-basal ganglia loop allows limbic control over motor output via the nigrothalamic pathway. eLife 8, e49995. (doi: 10.7554/eLife.49995.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Maren S, & McNally GP (2021). Behavioral and neurobiological mechanisms of Pavlovian and instrumental extinction learning. Physiological Reviews, 101, 611–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, & Quirk GJ (2007). Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. Journal of Neuroscience, 27(4), 840–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, & Whalen PJ (2001). The amygdala: vigilance and emotion. Molecular Psychiatry, 6(1), 13–34. [DOI] [PubMed] [Google Scholar]
- DeLong M, & Wichmann T (2009). Update on models of basal ganglia function and dysfunction. Parkinsonism & Related Disorders, 15, S237–S240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Monte FH, Quinones-Laracuente K, & Quirk GJ (2015). A temporal shift in the circuits mediating retrieval of fear memory. Nature, 519(7544), 460–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Li S, and Kirouac GJ (2017). Collateralization of projections from the paraventricular nucleus of the thalamus to the nucleus accumbens, bed nucleus of the stria terminalis, and central nucleus of the amygdala. Brain Struct. Funct 222, 3927–3943. doi: 10.1007/s00429-017-1445-8 [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Niv Y, Daw N, & Phelps EA (2015). Rethinking extinction. Neuron, 88(1), 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, & Shackman AJ (2019). The central extended amygdala in fear and anxiety: Closing the gap between mechanistic and neuroimaging research. Neuroscience Letters, 693, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wouterlood FG, Uylings HBM. 2016. Organization of prefrontal–striatal connections. In Handbook of basal ganglia structure and function (eds Steiner H, Tseng K), chapter 21, pp. 423–438, 2nd edn. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- Grupe DW, & Nitschke JB (2013). Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14(7), 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor NZ, & Paré D (2016). Functional heterogeneity in the bed nucleus of the stria terminalis. Journal of Neuroscience, 36(31), 8038–8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Weiner I. 1994. The organization of the basal ganglia–thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience 63, 363–379. (doi: 10.1016/0306-4522(94)90536-3) [DOI] [PubMed] [Google Scholar]
- Johansen JP, Tarpley JW, LeDoux JE, & Blair HT (2010). Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nature Neuroscience, 13(8), 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. 2010. Integrative networks across basal ganglia circuits. In Handbook of basal ganglia structure and function (eds Steiner H, Tseng K), chapter 24, pp. 409–427. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- Heimer L, Van Hoesen GW, Trimble M, & Zahm DS (2007). Anatomy of neuropsychiatry: the new anatomy of the basal forebrain and its implications for neuropsychiatric illness. Academic Press. [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, & Lüthi A (2008). Switching on and off fear by distinct neuronal circuits. Nature, 454(7204), 600–606. [DOI] [PubMed] [Google Scholar]
- Hintiryan H et al. 2016. The mouse cortico-striatal projectome. Nature Neuroscience, 19, 1100–1114. (doi: 10.1038/nn.4332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley D, & Fox AS (2022). The central extended amygdala guides survival-relevant tradeoffs: Implications for understanding common psychiatric disorders. Neuroscience & Biobehavioral Reviews, 104879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Smith JF, DeYoung KA, Anderson AS, Kuang J, Kim HC, … & Shackman AJ (2020). Anxiety and the neurobiology of temporally uncertain threat anticipation. Journal of Neuroscience, 40(41), 7949–7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac GJ (2015). Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neuroscience & Biobehavioral Reviews, 56, 315–329. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ (2021). The paraventricular nucleus of the thalamus as an integrating and relay node in the brain anxiety network. Frontiers in Behavioral Neuroscience, 15, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AS, Dolensek N, Weiand C, & Gogolla N (2021). Fear balance is maintained by bodily feedback to the insular cortex in mice. Science, 374(6570), 1010–1015. [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23(1), 155–184. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, & Lüthi A (2011). A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature, 480(7377), 331–335. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, & Gordon JA (2014). Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nature Neuroscience, 17(1), 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (2001). Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience, 24(1), 897–931. [DOI] [PubMed] [Google Scholar]
- Murty DV, Song S, Morrow K, Kim J, Hu K, & Pessoa L (2022). Distributed and Multifaceted Effects of Threat and Safety. Journal of Cognitive Neuroscience, 34(3), 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, & Kreitzer AC (2014). Reassessing models of basal ganglia function and dysfunction. Annual Review of Neuroscience, 37, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, & Quirk GJ (2017). When scientific paradigms lead to tunnel vision: lessons from the study of fear. npj Science of Learning, 2(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, & Stein MB (2006). An insular view of anxiety. Biological Psychiatry, 60(4), 383–387. [DOI] [PubMed] [Google Scholar]
- Pessoa L (2014). Understanding brain networks and brain organization. Physics of Life Reviews, 11(3), 400–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L (2022). The Entangled Brain. Journal of Cognitive Neuroscience, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Medina L, Hof PR, & Desfilis E (2019). Neural architecture of the vertebrate brain: implications for the interaction between emotion and cognition. Neuroscience & Biobehavioral Reviews, 107, 296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Medina L, & Desfilis E (2022). Refocusing neuroscience: moving away from mental categories and towards complex behaviours. Philosophical Transactions of the Royal Society B, 377(1844), 20200534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, & Fox AS (2016). Contributions of the central extended amygdala to fear and anxietycontributions of the central extended amygdala to fear and anxiety. Journal of Neuroscience, 36(31), 8050–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, & Lüthi A (2015). Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience, 16(6), 317–331. [DOI] [PubMed] [Google Scholar]
- Unzai T, Kuramoto E, Kaneko T, and Fujiyama F (2015). Quantitative analyses of the projection of individual neurons from the midline thalamic nuclei to the striosome and matrix compartments of the rat striatum. Cereb. Cortex 27, 1164–1181. [DOI] [PubMed] [Google Scholar]
- Yin HH, & Knowlton BJ (2006). The role of the basal ganglia in habit formation. Nature Reviews Neuroscience, 7(6), 464–476. [DOI] [PubMed] [Google Scholar]


