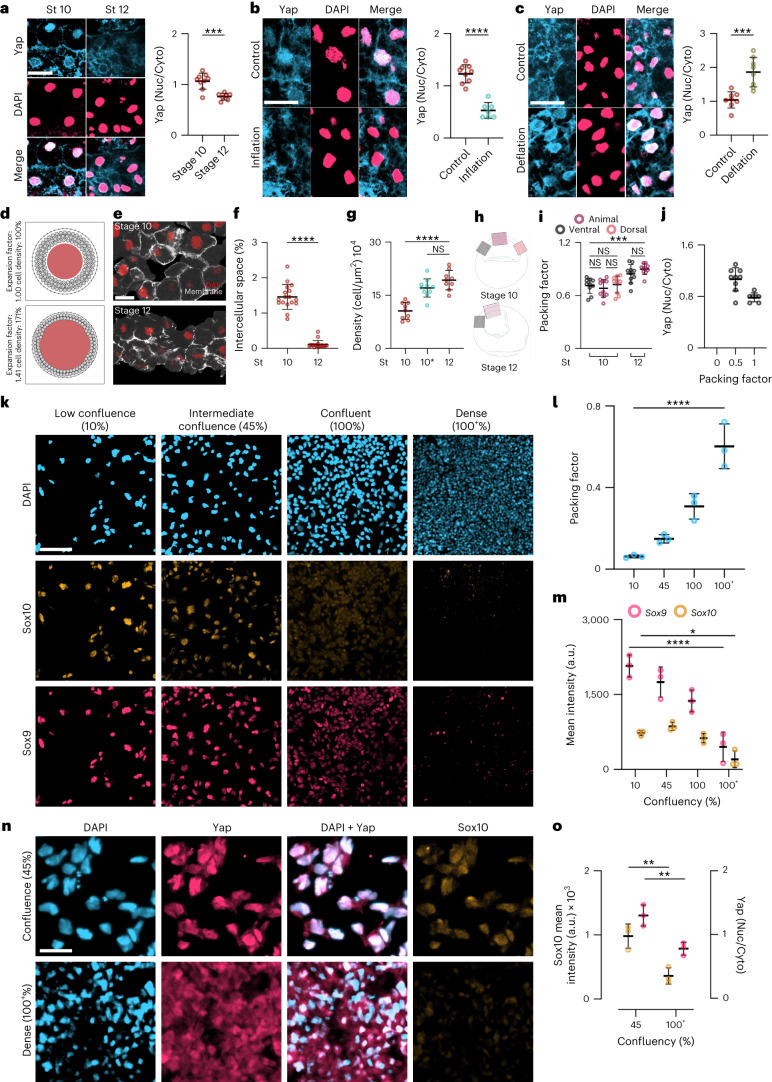
Fig. 5. Hydrostatic pressure controls Yap localization.
a–c, Immunofluorescence of ectoderm showing Yap (blue) and DAPI (pink) during gastrulation (a) inflation (b), and deflation (c); graphs show quantification of the Yap nuclear-to-cytoplasmic (Nuc/Cyto) ratio. d, Particle-based 2D model illustrating how an expanding cavity (red) can compress surrounding cells (light-grey circles) if they are encapsulated by a stiff shell (the vitelline membrane, dark-grey outer layer). Dark-grey connection lines between cells indicate adhesive interactions. e, Immunofluorescence sections of the ectoderm expressing membrane GFP (grey) and stained for DAPI (red). f,g, Spread of data showing the percentage of intercellular space (f) and density (g). 10* indicates inflated embryos at stage 10 (cyan) and control embryos (red) at the indicated stages. h, Schematic of embryos indicating the regions of interest of ectoderm for analysis of ectoderm packing quantified in i. j, Spread of data indicating the relationship of ectoderm packing and Yap localization. k, Immunofluorescence of different confluences of iNCCs expressing Sox9 (pink) and Sox10 (orange). l,m, Spread of data showing different cell packing and mean fluorescence intensity at different densities. n, Immunofluorescence of Yap (pink) and Sox10 (orange) localization at different confluences of iNCCs. o, Spread of data showing mean fluorescence intensity of Sox10 and Yap at different densities. Scale bars, 20 µm (a–c,e), 100 µm (k) and 65 µm (n). Data are mean and s.d. Statistical analysis was performed using a two-sided two-tailed Student’s t-test and Dunnett’s test; (NS, P ≥ 0.1408 (j,l), *P = 0.0168 (m), ***P ≤ 0.0010 (a–c,i) and ****P = 0.0001 (b,f,g,l,m), 95% CI). Three independent experiments (i,j,l,m,o). n = 9st10, 6st12 embryos (a), n = 9control, 7inflation embryos (b), n = 8control, 8deflation embryos (c), nst10 and nst12 = 20 embryos (f), and nst10 = 9, nst10* = 10, nst12 = 9 embryos (g).