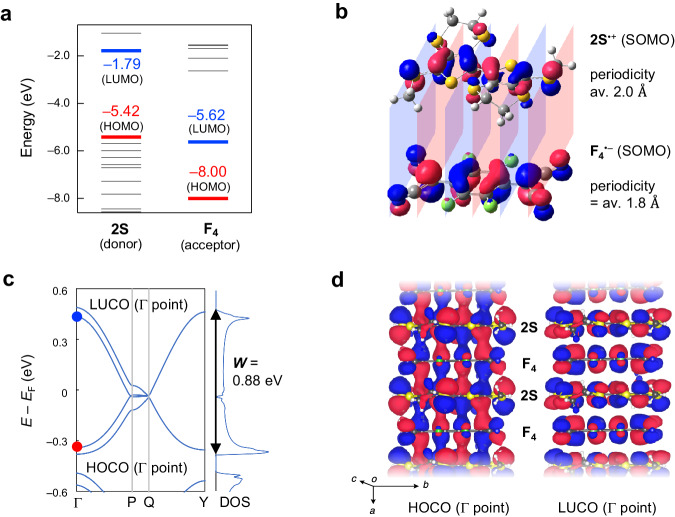
Fig. 4. Electronic structure of donor 2S, acceptor F4, and the mixed-stack complex 2S–F4 according to theoretical calculations.
a Energy levels of orbitals for neutral 2S donor and F4 acceptor calculated by the Gaussian09 program. The energy levels of the donor’s highest occupied molecular orbital (HOMO) and acceptor’s lowest unoccupied molecular orbital (LUMO) were comparable, which is appropriate for their strong hybridization. b Singly occupied molecular orbitals (SOMOs) of 1e–-oxidized 2S donor and 1e–-reduced F4 acceptor calculated by Gaussian09 program. These orbitals have horizontally nodal patterns with an average periodicity of 1.8–2.0 Å that correspond well with each other (Supplementary Fig. 5). c Band structure calculated by OpenMX38,39. The bandwidth (W) value was determined from the calculated density of states (DOS). The highest occupied crystal orbital (HOCO) and lowest unoccupied crystal orbital (LUCO) at the Γ-point are depicted in red and blue dots, respectively. Γ (0,0,0), P (–0.5,0,0.5), Q (–0.5,0.5,0.5), Y (0,0.5,0). d Highly hybridized HOCO and LUCO between donor and acceptor at the Γ-point calculated by OpenMX38,39. Atoms were colored as follows; white: hydrogen; gray: carbon; blue: nitrogen, red: oxygen; light green: fluorine; yellow: sulfur. Molecular and crystal orbitals with positive and negative phases were colored with magenta and navy, respectively.