Abstract
Mass mortality of the dominant coral reef herbivore Diadema antillarum in the Caribbean in the early 1980s contributed to a persistent phase shift from coral- to algal-dominated reefs. In 2022, a scuticociliate most closely related to Philaster apodigitiformis caused further mass mortality of D. antillarum across the Caribbean, leading to >95% mortality at affected sites. Mortality was also reported in the related species Diadema setosum in the Mediterranean in 2022, though the causative agent of the Mediterranean outbreak has not yet been determined. In April 2023, mass mortality of Diadema setosum occurred along the Sultanate of Oman's coastline. Urchins displayed signs compatible with scuticociliatosis including abnormal behavior, drooping and loss of spines, followed by tissue necrosis and death. Here we report the detection of an 18S rRNA gene sequence in abnormal urchins from Muscat, Oman, that is identical to the Philaster strain responsible for D. antillarum mass mortality in the Caribbean. We also show that scuticociliatosis signs can be elicited in Diadema setosum by experimental challenge with the cultivated Philaster strain associated with Caribbean scuticociliatosis. These results demonstrate the Philaster sp. associated with D. antillarum mass mortality has rapidly spread to geographically distant coral reefs, compelling global-scale awareness and monitoring for this devastating condition through field surveys, microscopy, and molecular microbiological approaches, and prompting investigation of long-range transmission mechanisms.
Keywords: Diadema, scuticociliatosis, ciliate, urchin, Philaster
The long-spined sea urchin genus Diadema is ubiquitous in tropical reef habitats across the globe, where it exerts critical control on algal growth [1], allowing sufficient light and space for new corals to settle and thrive [2, 3]. The loss of these important herbivores can result in phase shifts from coral- to algal-dominated communities, with widespread ecosystem effects [1, 4].
A mass mortality event of unknown etiology decimated Caribbean Diadema antillarum populations in the early 1980s, with very limited recovery in subsequent years [4-7]. Another D. antillarum mass mortality event was reported in February 2022 in the US Virgin Islands and by May 2022 abnormal urchins were observed across the Caribbean [7, 8]. The 2022 mass mortality was caused by a scuticociliate most closely related to Philaster apodigitiformis [8]. Signs of the condition, termed D. antillarum scuticociliatosis, include abnormal behavior, loss of tube foot control, stellate spine orientation, spine drooping and loss, and finally tissue necrosis and death [7, 8]. In both the 1980s and 2022 die-offs, no other echinoid species were reportedly affected [4, 8].
Beginning in July 2022, mass mortality was observed in clade b Diadema setosum in its invasive range in the Mediterranean Sea [9]. Signs resembled scuticociliatosis [8, 9], but the etiological agent was not determined. In April 2023, we observed abnormal clade b D. setosum in the Sea of Oman (Fig. 1). Abnormal individuals were collected and preserved in RNALater until DNA was extracted from urchin samples (~1-mm body wall fragments, one to three spines with bases, or 200-μl coelomic fluid) using the Zymo Quick-DNA Tissue/Insect Kit (Irving, CA, USA) following manufacturer’s instructions with the exceptions of omitting β-mercaptoethanol from the lysis buffer, bead-beating for 2 min, and eluting into sterile water. Urchin species and clade were confirmed through CO2b/ATP6b gene amplification and sequencing [10] (Fig. 2A). We amplified the Philaster clade associated with Diadema scuticociliatosis from six abnormal urchins collected from the Capital Area Yacht Club in Muscat, Oman, using nested PCR [11, 12]. Sequencing confirmed that these samples contained 18S rRNA gene sequences identical to P. apodigitiformis FWC2, the agent responsible for scuticociliatosis in the Caribbean [8] (Fig. 2B). FWC2 was cultured from the coelomic fluid of a D. antillarum specimen with scuticociliatosis in the Florida Keys in June 2022, and has been maintained in xenic culture.
Figure 1.
Abnormal D. setosum reported from the Sea of Oman. (A) Graphical depiction of locations in the Sea of Oman where D. setosum die-offs were reported (circles) and confirmed in collected individuals via PCR (diamonds). Inlaid images show more detailed maps of sites in Fujairah (left) and Muscat (top). Basemap created in QGIS using data freely available from a general bathymetric chart of the ocean (www.gebco.net) and EOX (maps.Eox.At). (B–E) images depicting signs seen in urchins considered “grossly abnormal,” including unusual behavior (B), stellate spine arrangement (C), spine loss and tissue necrosis (D), and eventual death (E). Photo credit: Kaileigh Cornfield.
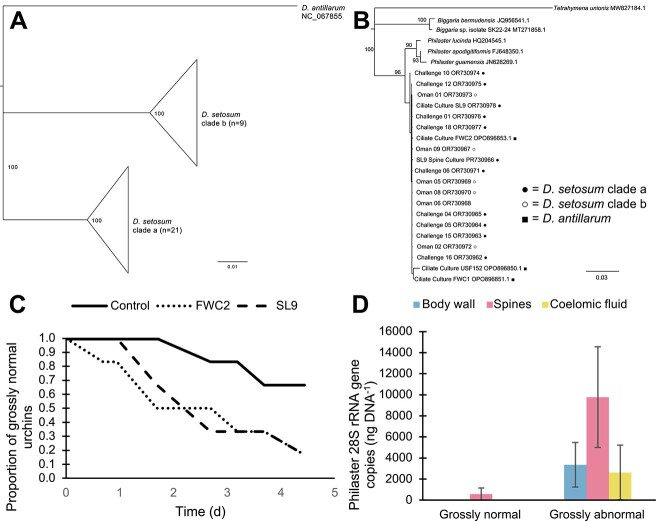
Figure 2.
Diadema and scuticociliate phylogenies and results of challenge experiments. (A) Phylogenetic representation of D. setosum clades a and b sampled during this experiment, as well as D. antillarum. (B) Phylogenetic representation of scuticociliate sequences from D. setosum (clade a = filled circle, clade b = empty circle), D. antillarum (filled square), and close relatives identified by BLASTn searches. All phylogenetic analyses were performed in Geneious prime using the Geneious tree builder and Tamura-Nei genetic distance model with the neighbor-joining method. (C) Survivorship curve showing the decrease in grossly normal individuals over time for urchins treated with two Philaster cultures (FWC2 and SL9) and the controls. (D) qPCR results for body wall, spines, and coelomic fluid samples from the 18 experimental challenge urchins, classified by animal state at the end of the experiment.
To determine if clade a D. setosum, native to the Indo-Pacific, is also susceptible to scuticociliatosis, we ordered specimens from commercial aquarium suppliers for use in challenge experiments. One urchin (designated SL9) presented scuticociliatosis signs upon arrival and we observed ciliates resembling P. apodigitiformis swarming in dropped spines under microscopy. Following the protocols previously applied to culture FWC2 [8], we established the SL9 culture from the coelomic fluid of this urchin. Within 48 h of incubation at room temperature, the SL9 culture was densely populated by ciliates morphologically similar to FWC2 and dilution-to-extinction was performed. PCR and sequencing confirmed 100% identity between the 18S rRNA gene sequences of the FWC2 and SL9 cultures (Fig. 2B).
Detecting this scuticociliate in an urchin obtained through the aquarium trade provided initial evidence for the ability of P. apodigitiformis to infect clade a D. setosum, leading us to conduct a controlled 5-day experimental challenge. Eighteen urchins acquired through aquarium suppliers (Fig. 2A) were placed into individual tanks filled with ~7 l of 5-μm-filtered Florida Keys seawater and an airstone bubbler. Twelve urchins were inoculated with ~250 ciliates each by addition to the water directly above the urchin (n = 6 FWC2, n = 6 SL9), and the remaining six urchins were treated with the same volume of 5-μm-filtered culture (n = 3 FWC2, n = 3 SL9) to control for bacteria within the media. Urchins were monitored for signs of infection and collected when disease was apparent or at experiment termination after 5 days; water used in challenge experiments was bleached for 24 h to kill any ciliates before disposal.
Upon collection, urchins were dissected to obtain coelomic fluid, spine/spine base, and body wall samples, which were frozen at -80°C until DNA extraction and quantitative PCR (qPCR) for P. apodigitiformis following previously published protocols [8]. Five of the six urchins treated with each ciliate culture lost spines and died, whereas only two of the six controls (one each of FWC2 and SL9 filtrate) exhibited signs of infection (Fig. 2C), likely resulting from ciliate exposure prior to arrival at our facility. Grossly normal urchins had lower levels of P. apodigitiformis in body wall, spine, and coelomic fluid samples than abnormal urchins by qPCR for the 28S rRNA gene, regardless of treatment (Fig. 2D). Although the negative impacts of scuticociliatosis on Diadema spp. are clear, many questions remain about the factors that affect P. apodigitiformis growth and pathogenicity, as well as Diadema immune responses and mechanisms for disease resistance. Although D. antillarum and D. setosum exhibit similar signs of scuticociliatosis, individuals of both species display variability in their responses to infection, including some experimentally infected specimens of each species that remained grossly normal.
Our experimental challenge results, combined with the detection of P. apodigitiformis in field samples from Oman, demonstrate that this parasite can cause scuticociliatosis in both clades of D. setosum, representing a significant threat to these important herbivores. Despite clade b D. setosum being an invasive species in the Mediterranean Sea, the detection of scuticociliatosis in the Sea of Oman, part of its native Red Sea habitat, could have disastrous ecological effects on coral reef communities reminiscent of those seen in the Caribbean following the 1980s die-off [1, 4, 6]. These results highlight the importance of monitoring urchin densities and ecosystem-level effects resulting from loss of these keystone herbivores in affected regions. Additionally, our experimental infection of clade a D. setosum indicates the vulnerability of this population to scuticociliatosis should the ciliate reach its native range, emphasizing the need for baseline benthic surveys. We also observed mortality of Echinothrix sp. in Fujairah and Al Fahal Island, suggesting that other species in the Diadematidae family may be susceptible to scuticociliatosis. Similar to the ciliate Philaster lucinda, which is associated with many coral diseases [13], P. apodigitiformis has now been detected in geographically disparate locations. Therefore, it is critical to assess long-range transmission routes of this ciliate, including natural (e.g. currents, seabirds) and anthropogenic (e.g. shipping vessels, recreational diving, aquarium trade) pathways.
Acknowledgements
Diadema setosum collected off the shore of Oman were collected under permit #6210/10/166 from the Sultanate of Oman. We thank Shen Jean Lim for help with sequence analysis. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Contributor Information
Isabella T Ritchie, College of Marine Science, University of South Florida, St. Petersburg, FL 33701, United States.
Brayan Vilanova-Cuevas, Department of Microbiology, Cornell University, Ithaca, NY 14853, United States.
Ashley Altera, Department of Microbiology, Cornell University, Ithaca, NY 14853, United States.
Kaileigh Cornfield, Five Oceans Environmental Services, Muscat 131, Oman.
Ceri Evans, Five Oceans Environmental Services, Muscat 131, Oman.
James S Evans, U.S. Geological Survey, St. Petersburg Coastal and Marine Science Center, St. Petersburg, FL 33701, United States.
Maria Hopson-Fernandes, College of Marine Science, University of South Florida, St. Petersburg, FL 33701, United States.
Christina A Kellogg, U.S. Geological Survey, St. Petersburg Coastal and Marine Science Center, St. Petersburg, FL 33701, United States.
Elayne Looker, Five Oceans Environmental Services, Muscat 131, Oman.
Oliver Taylor, Five Oceans Environmental Services, Muscat 131, Oman.
Ian Hewson, Department of Microbiology, Cornell University, Ithaca, NY 14853, United States.
Mya Breitbart, College of Marine Science, University of South Florida, St. Petersburg, FL 33701, United States.
Author contributions
Isabella T. Ritchie (designed the experiments, performed the experiments, drafted the initial manuscript, edited and approved the final submission), Brayan Vilanova-Cuevas (designed the experiments, performed the experiments, edited and approved the final submission), Ashley Altera (performed the experiments, edited and approved the final submission), Kaleigh Cornfield (collected field samples, drafted the initial manuscript, edited and approved the final submission), Ceri Evans (collected field samples, edited and approved the final submission), James S. Evans (designed the experiments, performed the experiments, edited and approved the final submission), Maria Hopson-Fernandes (performed the experiments, edited and approved the final submission), Christina A. Kellogg (designed the experiments, performed the experiments, edited and approved the final submission), Elayne Looker (collected field samples, edited and approved the final submission), Oliver Taylor (collected field samples, edited and approved the final submission), Ian Hewson (designed the experiments, performed the experiments, edited and approved the final submission), and Mya Breitbart (designed the experiments, performed the experiments, drafted the initial manuscript, edited and approved the final submission)
Conflicts of interest
None declared.
Funding
This work was supported by the Atkinson Center for Sustainable Futures Rapid Response Fund and OCE-2049225 to Ian Hewson and the Von Rosenstiel Fellowship and Von Rosenstiel Innovation Fund for Marine Science to Isabella T. Ritchie. Isabella T. Ritchie and Brayan Vilanova-Cuevas were supported by the NSF Graduate Research Fellowship Program (#2136515 and #1650441). James S. Evans and Christina A. Kellogg were supported by the U.S. Geological Survey Ecosystems Mission Area Biological Threats and Invasive Species Research Program.
Data availability
All ciliate sequences generated in this study are available in GenBank (Accession Numbers: OR730962-OR730978).
References
- 1. Carpenter RC. Mass mortality of Diadema antillarum: I. Long-term effects on sea urchin population-dynamics and coral reef algal communities. Mar Biol 1990;104:67–77. 10.1007/BF01313159. [DOI] [Google Scholar]
- 2. Williams SM. The reduction of harmful algae on Caribbean coral reefs through the reintroduction of a keystone herbivore, the long-spined sea urchin Diadema antillarum. Restor Ecol 2022;30:e13475. 10.1111/rec.13475. [DOI] [Google Scholar]
- 3. Idjadi J, Haring R, Precht W. Recovery of the sea urchin Diadema antillarum promotes scleractinian coral growth and survivorship on shallow Jamaican reefs. Mar Ecol Prog Ser 2010;403:91–100. 10.3354/meps08463. [DOI] [Google Scholar]
- 4. Lessios HA. The great Diadema antillarum die-off: 30 years later. Annu Rev Mar Sci 2016;8:267–83. 10.1146/annurev-marine-122414-033857. [DOI] [PubMed] [Google Scholar]
- 5. Lessios HA, Robertson DR, Cubit JD. Spread of Diadema mass mortality through the Caribbean. Science 1984;226:335–7. 10.1126/science.226.4672.335. [DOI] [PubMed] [Google Scholar]
- 6. Lessios HA. Mass mortality of Diadema antillarum in the Caribbean: what have we learned? Annu Rev Ecol Syst 1988;19:371–93. 10.1146/annurev.es.19.110188.002103. [DOI] [Google Scholar]
- 7. Hylkema A, Kitson-Walters K, Kramer PR et al. The 2022 Diadema antillarum die-off event: comparisons with the 1983-1984 mass mortality. Front Mar Sci 2023;9:1067449. 10.3389/fmars.2022.1067449. [DOI] [Google Scholar]
- 8. Hewson I, Ritchie IT, Evans JS et al. A scuticociliate causes mass mortality of Diadema antillarum in the Caribbean Sea. Sci Adv 2023;9:eadg3200. 10.1126/sciadv.adg3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zirler R, Schmidt LM, Roth L et al. Mass mortality of the invasive alien echinoid Diadema setosum (Echinoidea: Diadematidae) in the Mediterranean Sea. R Soc Open Sci 2023;10:230251. 10.1098/rsos.230251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lessios HA, Kessing BD, Pearse JS. Population structure and speciation in tropical seas: global phylogeny of the sea urchin Diadema. Evolution 2001;55:955–75. 10.1554/0014-3820(2001)055[0955:PSASIT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11. Vilanova-Cuevas BY, Reyes-Chavez B, Breitbart M et al. Design and validation of a PCR protocol to specifically detect the clade of Philaster sp. associated with Diadema antillarum scuticociliatosis. BioRxiv 2023; 10.1101/2023.09.11.557215. [DOI] [Google Scholar]
- 12. Dopheide A, Lear G, Stott R et al. Molecular characterization of ciliate diversity in stream biofilms. Appl Environ Microbiol 2008;74:1740–7. 10.1128/AEM.01438-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sweet MJ, Séré MG. Ciliate communities consistently associated with coral diseases. J Sea Res 2016;113:119–31. 10.1016/j.seares.2015.06.008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All ciliate sequences generated in this study are available in GenBank (Accession Numbers: OR730962-OR730978).