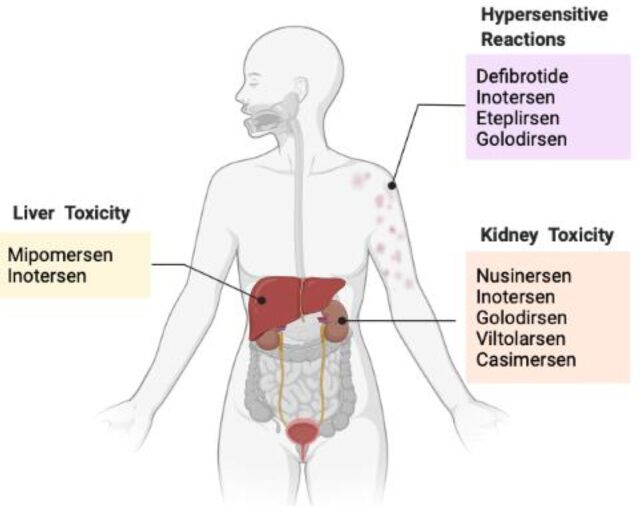
Fig. 1.
Shared warnings of toxicity in multiple FDA-approved ASO drugs. Hypersensitive reactions, such as rash and urticaria, are shown in defibrotide, inotersen, eteplirsen, and golodirsen. Hepatotoxicity is indicated after treatment with mipomersen and inotersen. Kidney toxicity is shown with the treatment of nusinersen, inotersen, golodirsen, viltolarsen, and casimersen.